New technique improves accuracy of near-infrared fluorescence imaging
Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are the two most common intravascular imaging systems used in cardiac cath labs to offer detailed views inside coronary vessels. However, there are limitations to these technologies and the plaque morphology information they can provide. This has led to the development of a new type of imaging called near-infrared fluorescence (NIRF) that uses injected biofluorecence dyes to clearly identify thrombus and the types of plaques within the vessel wall.[1]
Plaque assessments based on pathobiology, a key driver of coronary events, are on the verge of clinical translation to human coronary arteries. Researchers are trying to perfect NIRF so it can provide visualization of pathobiological and cellular processes at atheroma plaque level, including inflammation, oxidative stress and abnormal endothelial permeability.
NIRF uses injected fluorescent agents that can show biological processes inside the body by binding to specific pathology-related compounds on vessel walls, such as proteins or nucleic acids. NIRF imaging is combined with IVUS to create hybrid NIRF-IVUS measurements. These allow co-registered imaging of morphological and molecular processes with a single catheter pullback.
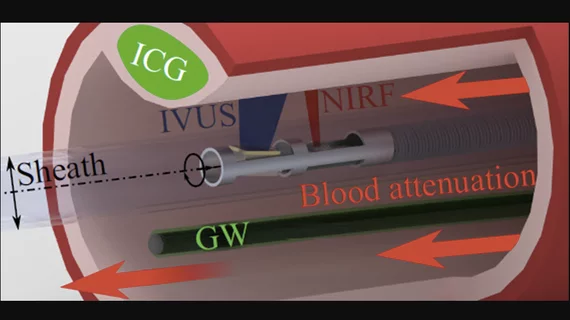
However, researchers found a big engineering problem for accuracy is the distance between the NIRF detector and the blood vessel wall keeps changing while the probe is stationary. The changing measurements are due to blood attenuating the intensity of the fluorescence signals, and the amount of blood between the NIRF detector and the vessel wall varies constantly.
Solving the problem on blood attenuation causing inaccurate NIRF measurements
A team of researchers led by Professor Vasilis Ntziachristos from the Technical University of Munich in Germany came up with an innovative solution to this problem in a study published in Journal of Biomedical Optics (JBO), the journal of the Society of Photographic Instrumentation Engineers (SPIE).
The guidewire is always visible to the NIRF probe, so coating the guidewire with a known concentration of fluorescent particles ensures that the signal on the guidewire will provide an indirect measure of the blood attenuation in the current image. IVUS is used to determine the distance between the NIRF probe and the guidewire, and the distance between the NIRF probe and the blood vessel wall. A correction factor for the fluorescence signal measured at the blood vessel wall can be calculated on each frame after a simple calibration procedure.
“We provide an adaptive correction scheme tailored to each patient and each imaging frame collected during the imaging procedure,” Ntziachristos said in a statement.
The researchers used a prototype NIRF-IVUS system to test their technique in a clinical model. They also performed experiments on capillary phantoms, which simulate the properties of small blood vessels. They recorded a 4.5-fold improvement over uncorrected NIRF signal and less than 11 percent errors for target signals. The correction method also maintained a mean accuracy of 70 percent in tissue experiments. These values are are much better than the accuracies obtained by other correction methods that averaged attenuation factors.
The team suggests that it should be relatively easy to directly incorporate their technique into clinical practice, since no major modifications to existing equipment are required.
“This new method for correcting intravascular NIRF signals is simple and accurate and could pave the way for in vivo studies and eventual clinical translation,” JBO Editor-in-Chief Brian Pogue, PhD, professor of medical physics at the University of Wisconsin – Madison, said in the same SPIE statement.
Clinical use of NIRF-IVUS imaging on then horizon to enable personalized care
Those involved in the development of NIRF imaging say the technology is almost ready for clinical use.They said this will be accelerated by the use of the FDA-approved indocyanine green (ICG) biofluorecence agent, which illuminates lipid- and macrophage-rich zones of permeable atheroma.
"The ability to comprehensively phenotype coronary pathobiology in patients will enable a deeper understanding of plaque pathobiology, improve local and patient-based risk prediction, and usher in a new era of personalized therapy," Haitham Khraishah, MD, and Farouc A. Jaffer, MD, who are both involved in the Massachusetts General Hospital Cardiovascular Research Center, wrote in a 2020 study assessment of the NIRF technology.
The review said IVUS only enables partial assessment of atheroma burden and high-risk features associated with acute coronary syndromes, but cannot phenotype the complex pathobiology of atherosclerosis. Jaffer said NIRF targets include macrophages, cathepsin protease activity, oxidized low-density lipoprotein and abnormal endothelial permeability, and by combining with IVUS it offers complementary information about plaque microstructure and biology.