FDA grants speedy review to Merck's new drug for pulmonary arterial hypertension
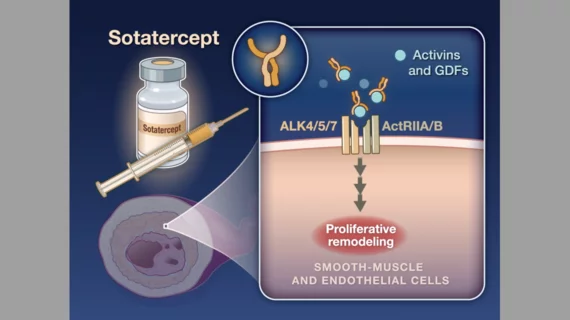
The U.S. Food and Drug Administration (FDA) has agreed to grant a priority review to Merck’s application for sotatercept, a newly developed activin signaling inhibitor, to be approved for the treatment of pulmonary arterial hypertension (PAH).
Priority reviews are much faster than the standard review process; they are typically reserved for drugs that, if approved, would lead to “significant improvements” in patient care.
Sotatercept would be the first drug in its class if approved for treating PAH. The FDA is expected to base its decision in part on data from the STELLAR clinical trial, which compared 163 adult PAH patients treated with subcutaneous sotatercept every three weeks, with another 160 patients treated with a placebo.
The study’s primary endpoint, the change in six-minute walk distance after 24 weeks of treatment, was 34.4 m among the sotatercept group and 1 m in the placebo group. Sotatercept was also associated with significant improvements in several secondary endpoints. The full results of the STELLAR trial were published in the New England Journal of Medicine back in March.[1]
“Despite advances in the treatment of PAH over the last two decades, there is still a significant need to improve outcomes for patients,” Joerg Koglin, MD, PhD, senior vice president of global clinical development for Merck Research Laboratories, said in a prepared statement. “The FDA’s acceptance of this application is an exciting milestone in our journey to bring this novel activin signaling inhibitor to patients. Based on the profound improvements across primary and secondary outcome measures in the Phase 3 STELLAR trial, we believe sotatercept has the potential to transform the treatment of patients with PAH. We look forward to working closely with the FDA to bring sotatercept to patients in need.”
Sotatercept previously received the FDA’s Breakthrough Therapy designation and Orphan Drug designation. It is the subject of a licensing agreement with Bristol Myers Squibb.