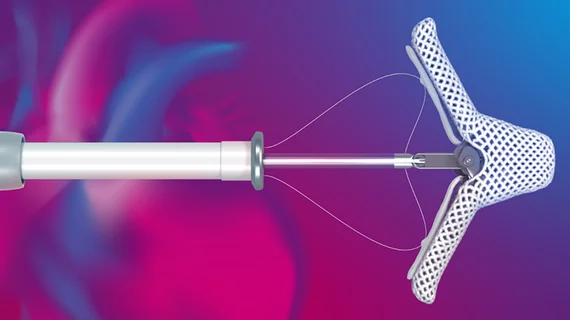
FDA warns of a new malfunction risk with Abbott’s MitraClip devices
The U.S. Food and Drug Administration (FDA) has shared a new warning related to potential issues with Abbott’s portfolio of MitraClip devices. The device delivery systems, it seems, have been associated with an increased number of clip lock malfunctions; clips are opening when they should be locked and/or moving unexpectedly following deployment.
“The potential risk to patients in the event of a clip lock malfunction includes ineffective treatment of mitral regurgitation (MR) and the potential need for additional interventions contributing to increased procedural risks such as bleeding, complications with implanting additional clips, and longer procedural times,” according to the FDA’s advisory. “The majority of reported clip lock malfunction events have not been associated with adverse patient outcomes.”
These malfunctions are currently being seen in approximately 1.3% of all MitraClip procedures. They have not led to any “immediate open surgical conversions,” but a very small percentage of malfunctions have led to “non-urgent open surgical conversions based on clinical decisions by the treating physician.”
At this point, the FDA emphasized, it is believed that the benefits of treatment with the MitraClip devices outweigh any potential risks. There is no need to return any devices to Abbott, though all incidents should be reported to MedWatch, the FDA Safety Information and Adverse Event Reporting program.
The FDA said it is important for all healthcare providers to know about these ongoing issues. Abbott’s Urgent Medical Device Correction details the issue at length and includes recommendations that should minimize the risk of a malfunction taking place.
“The FDA is working with the manufacturer to continue to evaluate reports of clip lock malfunctions and identify other potential contributing factors and mitigation strategies,” according to the agency. “The FDA will continue to monitor reports of adverse events related to the issue. The FDA will keep health care providers and the public informed if new or additional information becomes available.”
Read more coverage focused on the mitral valve and procedures such as transcatheter edge-to-edge repair/transcatheter mitral valve repair here.