SCAI and HRS release expert consensus on transcatheter left atrial appendage occlusion
To help standardize transcatheter left atrial appendage closure (LAAC) procedures, an updated expert consensus statement was released by the Society for Cardiovascular Angiography and Interventions (SCAI) and the Heart Rhythm Society (HRS) March 27.
Both the SCAI and HRS said they prioritized the development of an updated consensus statement to provide recommendations that are based on contemporary, evidence-based best practices for implanting transcatheter LAA occlusion (LAAO) devices. Additional clinical study data and user experience with recently introduced technologies contributed new information for the document, which was last updated in 2016.
Left atrial appendage closure is a minimally invasive procedure that is used to reduce the risk of stroke associated with atrial fibrillation (AFib). The arrhythmia is associated with a four- to five-fold increased risk of ischemic stroke, and accounts for 25% of the 700,000 cerebrovascular accidents that occur in the United States annually.
“This consensus statement demonstrates the evolvement of LAAC treatment since our first statements that were issued in 2015 and 2016,” said Jacqueline Saw, MD, FSCAI, chair of the writing group in a statement. She is an interventional cardiologist at Vancouver General Hospital and St Paul’s Hospital, clinical professor of medicine at UBC, and program director of the VGH interventional cardiology fellowship program. “Since then, the results from several important clinical trials and registries, as well as other technological and clinical advancements have evolved and changed the way we look at operator requirements, patient selection, and shared decision making, which explains the need for this updated guidance.”
Key takeaways and new information in the new consensus document include these sections:
1. Transcatheter LAAC is appropriate for patients with nonvalvular AFib with high thromboembolic risk who are not suited for long-term oral anticoagulation and who have adequate life expectancy.
2.1. Physicians performing LAAC should have prior experience, including ≥50 prior left-sided ablations or structural procedures and ≥25 transseptal punctures (TSPs). Interventional imaging physicians should have experience in guiding ≥25 TSPs before supporting any LAAC procedures independently.
2.2. For maintenance of skills, implanting physicians should perform ≥25 TSPs and >12 LAACs over each 2-year period.
2.3. New programs and implanting physicians early in their LAAC experience should have on-site cardiovascular surgery backup.
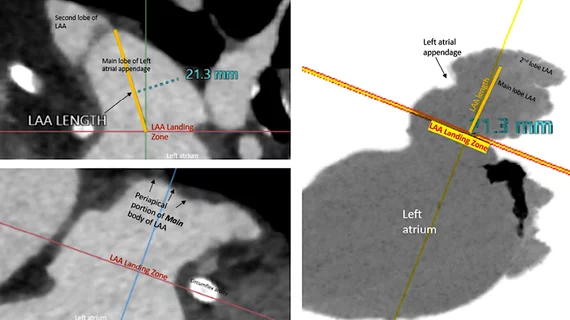
3. Baseline imaging with transesophageal echocardiography (TEE) or cardiac computed tomography (CT) is recommended before LAAC.
4. Intraprocedural imaging guidance with TEE or intracardiac echocardiography (ICE) is recommended.
5. Technical aspects of the procedure, including venous access, anticoagulation, transseptal puncture, delivery sheath selection and placement, left atrial pressure measurement, and device deployment, should be performed in accordance with the labeling of each specific LAAC device.
6. Operators need to be familiar with avoidance, recognition and management of procedural complications associated with LAAC.
7. Predischarge imaging should be performed with 2D transthoracic echocardiography to rule out pericardial effusion and device embolization.
8. Device-related thrombus should be treated with anticoagulation.
9. Routine closure of iatrogenic atrial septal defects associated with LAAC should not be performed.
10. The clinical impact and management of peridevice leaks are not fully understood, and all efforts should be made to minimize such leaks at the time of implantation.
11. Patients should be prescribed antithrombotic therapy with warfarin, direct oral anticoagulants, or dual antiplatelet therapy after LAAC according to the studied regimen and instructions for use for each specific device and tailored to the bleeding risks of each patient.
12. TEE or cardiac CT is recommended at 45 to 90 days after LAAC for device surveillance to assess for peridevice leak and device-related thrombus.
13. Combined procedures with LAAC (e.g., structural interventions, pulmonary vein isolation) are not routinely recommended, as data are pending from ongoing randomized controlled trials.
Debate about pre-procedural imaging form LAA occlusions
One of the new recommendations is to conduct baseline imaging with TEE or cardiac CT, which may help put to rest debate in the interventional community over whether pre-procedural imaging is really needed and worth the extra cost. This was a debate at the 2022 Transcatheter Valve Therapies (TVT) Structural Heart Summit last summer. Saw was among a panel of key LAA experts who discussed whether CT or pre-procedural TEE was needed during one of the LAA tract sessions. There was debate by some operators if pre-procedural CT was required. Some operators said they could just image the LAA during the procedure with TEE or ICE to get the measurements then needed to size a device. The panel was split with those favoring pre-procedural CT and those just using peri-procedural imaging.
Part of this boiled down to cost, where CT for pre-planning a procedure is not always covered. Some of the panelists said a CT might be helpful for inexperienced operators who only perform a few of these procedures a year, but for experienced operators it was not needed and echo alone could be used. Operators who support CT said it is very accurate and sizing on echo can sometimes lead to undersizing the LAA device, which can lead to peridevice leaks or device embolization.
Saw said she favored cardiac CT imaging for all her LAA patients because she wants to verify there is no thrombus in the LAA prior to the procedure that could embolize. She also said CT imaging offers more precise sizing for devices, where she uses the maximum dimension for determining what size device to use.
One of the co-authors of the consensus document is Dee Dee Wang, MD, director of structural heart imaging at Henry Ford Hospital. She recently conducted a study showing CT use was associated with higher successful device implantation rates, shorter procedural times, and less frequent changes in device sizes. Henry Ford also uses CT imaging to create 3D printed hearts and computer simulation models to take a deeper dive in patients with more complex anatomy.
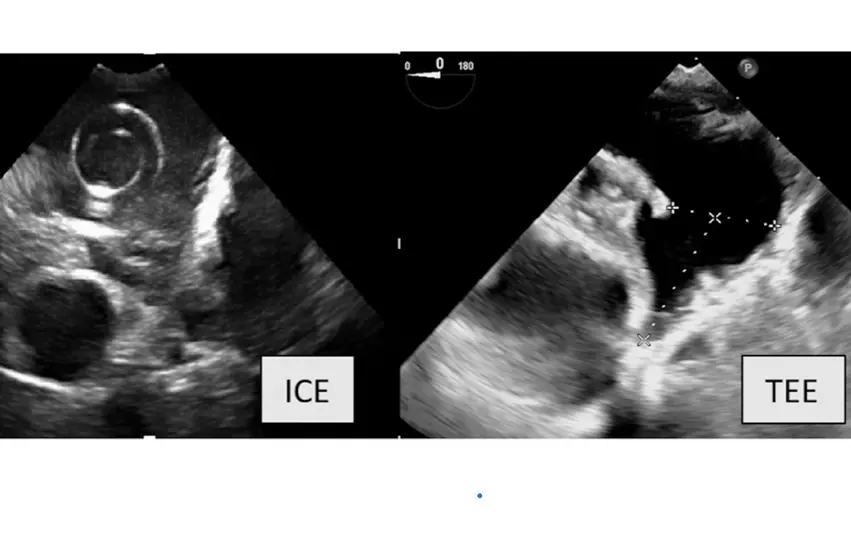
TEE was a standard-of-care for LAA procedural image guidance until recently. Some operators said they now opt for using 3D/4D ICE because it eliminates the need for an anesthesiologist and allows for conscience sedation. Since the operator is the one guides the ICE probe, it also might be possibly eliminate the need for a dedicated interventional echocardiographer. 4D ICE imaging quality has now improved enough over the old 2D technology that users say it is nearly as good as 3D/4D TEE.
The new consensus document also suggests use of TEE or cardiac CT at 45 to 90 days after LAAO for device surveillance to assess for peridevice leak and device-related thrombus.
A comparison of imaging of the same LAA anatomy between intra-cardiac echo (ICE) and transesophageal echo (TEE) in the new SCAI and HRS LAA occlusion consensus document. Some operators are using 3D/4D ICE to guide procedures and to eliminate the need for an anesthesiologist and the ability to use conscience sedation.
Imaging to check for LAA Occlusion complications
The authors of the consensus statement said device-related thrombosis (DRT) has been reported in between 3% to 5% of LAAC procedures in large clinical trial and registry experiences. They said DRT has been shown to be more likely in patients with prior stroke, large LAA dimensions, those with permanent AF, and those with previous LA thrombus. Registry analyses found an association between DRT and stroke/transient ischemic attack (TIA) during short-term follow-up, but most strokes after LAAC occur in patients without DRT.
Peridevice leaks (PDLs) are common and are reported in 11% to 57% of transcatheter LAA occlusion procedures depending on the implanted device and imaging modality used, the authors wrote. An analysis of 51,333 patients in the NCDR LAAO Registry found that small leaks of 5 mm are believed to be significant, and current practice is generally to continue OAC. Case reports and small case series have demonstrated the feasibility of closing leaks using interventional approaches including placement of occlusion or plug devices or coiling, but data are lacking on the long-term impact
Late pericardial effusion has been reported in 1% of patients in the PRAGUE-17 trial, which may be related to the hooks of the devices penetrating through the LAA wall. Management is similar to periprocedural pericardial effusion.
Rarer complications include device erosion, infection and nickel allergy reactions.
Two devices for LAA occlusion are now on the U.S. market
The U.S. Food and Drug Administration (FDA) cleared the Boston Scientific Watchman device in March 2015. The improved Watchman FLX device was cleared in July 2020.
A competitor, the Abbott Amplatzer Amuluet device came was cleared by the FDA in October 2021.
Both devices close off the appendage permanently to prevent the formation and embolization of blood clots in the LAA cause by AFib. The therapy gives patients with non-valvular atrial fibrillation an alternative option to life-long anticoagulation therapy.
LAAC can be conducted by an interventional cardiologist or an electrophysiologist.