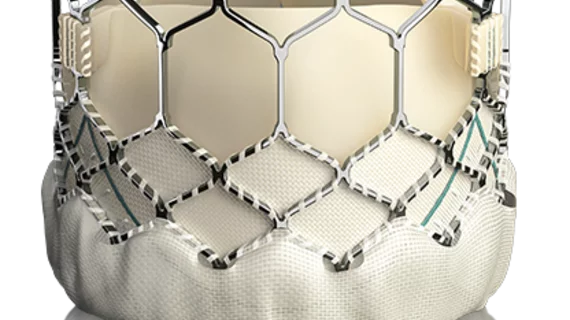
SAPIEN 3 valve cleared for use in low-risk patients in Europe
Edwards Lifesciences announced Nov. 6 that it had received expanded CE mark approval in Europe for the use of its SAPIEN 3 transcatheter heart valve, a tool designed to facilitate transcatheter aortic valve implantation (TAVI) in patients who don’t want to undergo open-heart surgery.
The SAPIEN 3 valve is the first TAVI system to receive a low-risk indication in Europe, according to a statement from Edwards, allowing patients who aren’t necessarily at a heightened risk for surgery to opt out of more invasive treatment.
“Now, all European patients diagnosed with aortic stenosis can be considered for TAVI with the SAPIEN 3 valve based on factors such as anatomical considerations or individual needs rather than risk scores,” Helge Mollmann, director of the Clinic for Internal Medicine at St. Johannes Hospital in Dortmund, Germany, said in the statement. “This is particularly important for patients at low risk for surgery, whose only serious health issue may be aortic stenosis and who want to return to their lives more safely and quickly. Previously, their only treatment option was open-heart surgery.”
The CE mark expansion is reportedly based on results from the PARTNER 3 trial, an Edwards-funded study that found TAVI with the SAPIEN 3 lowered low-risk patients’ odds of one-year all-cause mortality, stroke and rehospitalization by 46% compared to similar patients treated with open-heart surgery. An additional study, published in the Journal of the American College of Cardiology, revealed that SAPIEN 3 patients’ quality of life was superior to their peers treated with surgery one year after implantation.
According to Edwards, SAPIEN TAVI valves are the most widely studied transcatheter valves in the world, with more than 30,000 patients treated in clinical trials and registries across 65 countries. The SAPIEN valve first received approval in Europe in 2007, but its more advanced update, the SAPIEN 3 TAVI system, wasn’t approved for high- and intermediate-risk patients until 2014.