Abbott receives FDA approval for new self-expanding TAVR system
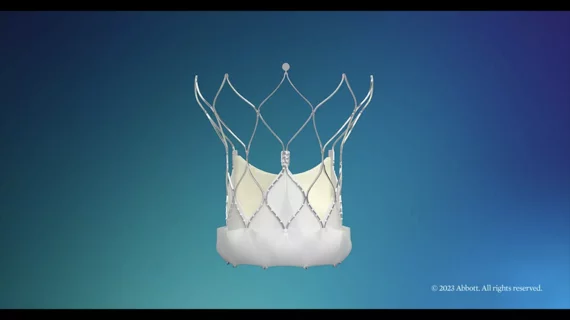
Abbott has gained U.S. Food and Drug Administration (FDA) approval for its Navitor transcatheter aortic valve replacement (TAVR) system, the company’s new self-expanding valve for high-risk patients with severe symptomatic aortic stenosis.
The Navitor system, which gained CE mark approval in Europe in May 2021, features a new-look fabric cuff sealing skirt designed to reduce the risk of paravalvular leak (PVL). According to Abbott, 80% of patients who receive the valve show no PVL, or only trace PVL, after 30 days. No patients presented with moderate or severe PVL.
The system also has the leaflets located at the same level as the native valve, a feature that Abbott has said improves access to the patient’s coronary arteries, The valve also uses a custom-designed anticalcification technology.
“Abbott's Navitor device features advancements to help doctors safely and effectively treat patients with aortic stenosis, including a design that reduces the backflow of blood around the valve that's often a complication following TAVR procedures,” Michael Reardon, MD, Alison Family Distinguished Chair of Cardiovascular Research and professor of cardiothoracic surgery at Houston Methodist Hospital, who has extensive experience evaluating the valve, said in a prepared statement. “The innovative Navitor system also offers physicians stable and accurate device placement, even in challenging patient anatomies.”
“Our Navitor valve builds upon our industry-leading portfolio of minimally invasive devices that surpass existing standards of care to address a range of heart diseases,” added Michael Dale, senior vice president of Abbott's structural heart business. “Navitor is the first TAVR system to offer optimal hemodynamics in all valve sizes, while also preserving options for lifetime disease management, an important consideration for physicians and patients when selecting a TAVR solution. Receiving this approval is a major next step in our mission to help people live better lives through better health."
The Navitor valve was designed to be implanted using Abbott’s FlexNav delivery system, which includes a “highly flexible” catheter and can be implanted in vessels as small as 5 mm.
Additional information about the newly approved solution is available here.