Cardiologists with the University Hospital of Cologne in Köln, Germany, performed the first transcatheter aortic valve replacement (TAVR) procedure of its kind, detailing their experience in JACC: Case Reports.[1]
The TAVR patient was a 65-year-old male who presented with a history of recurrent cardiac decompensation, exhaustion and dyspnea due to valvular heart failure with preserved ejection fraction (HFpEF). Using echocardiography, the care team determined severe aortic regurgitation (AR) was the “only valvular pathology.”
The patient’s medical history also included single-vessel coronary artery disease, peripheral arterial disease (PAD), chronic obstructive pulmonary disease, multiple heart failure hospitalizations and impaired mobility. His PAD had previously been treated with bi-iliac stenting. The Society of Thoracic Surgeons score was 5.32%.
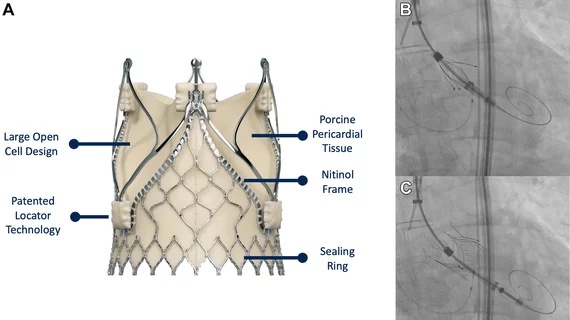
With this long list of prior health issues and comorbidities in mind, the care team determined surgery was not a realistic treatment option. They decided to perform TAVR with the self-expandable JenaValve Trilogy Heart Valve System, which first launched in Europe in 2021. The JenaValve device includes a nitinol stent frame with locators that help ensure correct commissural and axial alignment, the authors explained.
When planning for the TAVR procedure, the cardiologists found that their options were limited for a variety of reasons.
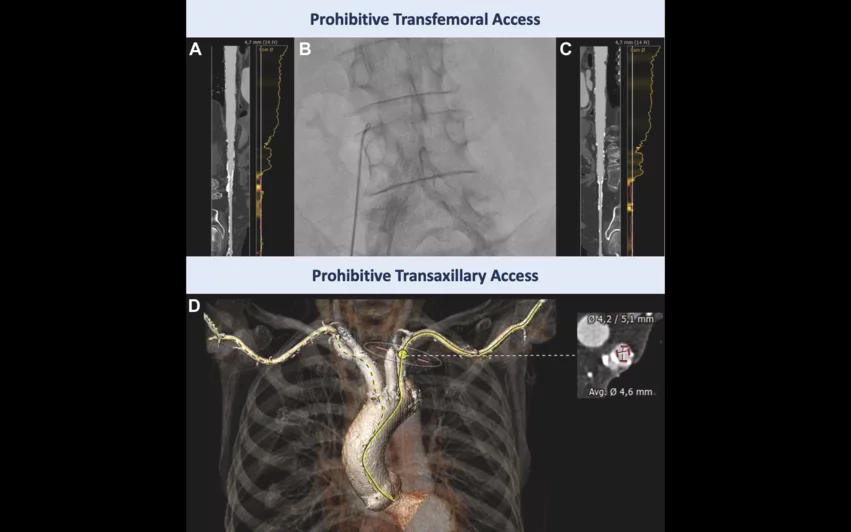
“Computed tomography angiography showed unfeasible transfemoral access, primarily due to the previously implanted bi-iliac stents,” wrote first author Jonathan Curio, an interventional cardiologist with the department of cardiology at the University of Cologne, and colleagues. “A transaxillary approach was abandoned as vessel diameters on both sides were too small (4.6 mm) and calcified. Transcarotid access was considered suboptimal owing to moderate stenotic disease of the vessel potentially bearing an increased stroke risk, leaving a transcaval access as a reasonable option. Importantly, with the delivery system of the novel self-expanding clippable device, alternative access must be considered carefully because the valve is implanted via a preshaped and 85-cm-long extended sheath. The preshaped distal sheath part must be placed just above the sinotubular-junction (STJ).”
Curio et al. identified a calcium-free area of the right aortic wall to plan their transcaval approach. The targeted location was not close to any crucial arterial branches, and the sheath’s trajectory was free of interposed bowel tissue.
The TAVR procedure required “meticulous positioning” of the guiding catheter, and a coaxial crossing system was electrified at 50 W during guidewire advancement. After advancing the introducer sheath, crossing the aortic valve and placing a Safari wire in the left ventricle, the team was able to move the delivery system with the loaded TAVR valve to the STJ. Angiographic control told the team valve placement was a success, with no signs of paravalvular leak. A nitinol duct occluder was then used to close the transcaval puncture site.
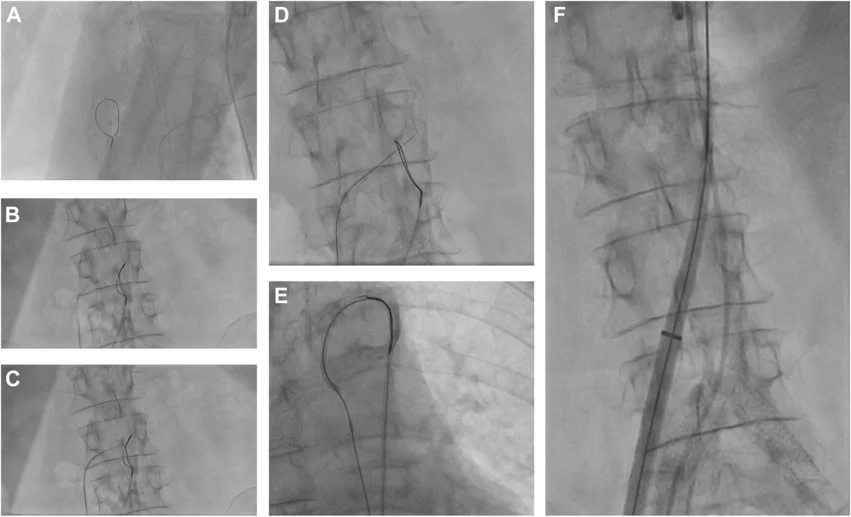
Transcaval crossover from vena cava to abdominal aorta. A loop snare positioned in the aorta and a crossing system placed in the vena cava (A,B). The electrified wire was advanced toward the abdominal aorta (C), where it was snared (D). The snared system was exchanged for a stiff guidewire and the delivery system of the self-expanding clippable device was advanced using the established transcaval access (E, F). Images/caption courtesy of Curio et al., JACC: Case Reports.
“This report describes the first case of transcaval access to treat pure AR with a dedicated self-expanding clippable device, resulting in excellent mid-term outcomes,” the authors wrote. “The transcaval approach was recently introduced as safe alternative access option, with low risk of stroke, transient ischemic attack, and similar rates of bleeding compared to an axillary access in the setting of TAVR for aortic stenosis.”
The cardiologists did warn that future care teams performing similar procedures may find that maneuverability of the TAVR valve is “impaired.” In addition, they said AR patients tend to “present with more tortuous and dilated aortas” than aortic stenosis patients.
Another takeaway following the procedure was that operators were required to perform multiple exchanges from a shorter sheath to the device’s actual sheath and back again.
“This might imply higher bleeding and rupture risks and needs to be carefully evaluated,” the authors wrote.
After three months, the patient reported a “substantial relief of symptoms” and key improvements in quality of life. CT scans confirmed full closure of the aortocaval fistula.
Read the full case report here.