Myval TAVR valve noninferior to similar devices from Medtronic, Edwards after 30 days
The balloon-expandable Myval transcatheter heart valve is associated with short-term transcatheter aortic valve replacement (TAVR) outcomes that are noninferior to similar devices from Medtronic and Edwards Lifesciences, according to new data presented at EuroPCR 2024 in Paris.
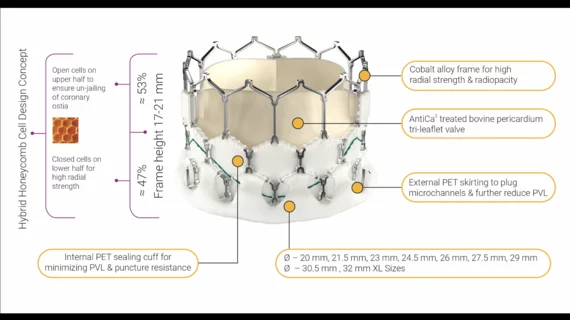
Meril Life Sciences, an India-based medical device company founded in 2006, has been manufacturing the Myval heart valves for years. They are already approved and available in both India and Europe. Meril developed the valves hoping that they could be seen by interventional cardiologists around the globe as a safe, effective alternative to the self-expanding Evolut TAVR systems manufactured by Medtronic and the balloon-expandable Sapien 3 TAVR systems manufactured by Edwards.
Meril designed the Myval valves in several sizes, a move the company says was intended to keep cardiologists from implanting a device that does not fit properly. It is available in 20 mm, 21.5 mm, 23 mm, 24.5 mm, 26 mm, 27.5 mm and 29 mm.
The LANDMARK trial included data from 768 low-risk patients undergoing transfemoral TAVR for severe aortic stenosis. Patients were randomized 1:1 to either receive a Myval TAVR valve or a contemporary TAVR valve from either Medtronic or Edwards.
After 30 days, the study’s primary composite endpoint—which included all-cause mortality, stroke, life-threatening or disabling bleeding, stage 2 or 3 acute kidney injury, major vascular complications, moderate or severe paravalvular leak (PVL) or the implantation of a permanent pacemaker—was seen in 24.7% of patients from the Myval group and 27.6% of patients from the contemporary group. There were no significant differences in any of the individual components of that endpoint. Also, technical and device success were both comparable between the two patient groups.
According to the study’s authors, these findings suggest the new Myval TAVR valves are noninferior to the Evolut and Sapien 3 devices, which are recognized as the gold standard of TAVR care throughout the United States.
“The LANDMARK trial showed that the Myval transcatheter heart valve series performed as safe and effective as contemporary transcatheter heart valve series,” principal investigator Andreas Baumbach, MD, chair of device innovation with Queen Mary University of London and a consultant cardiologist with Cleveland Clinic London, said in a statement. “It is a valve made for everyday clinical practice and an all-comers population. The special feature of intermediate diameters allows for more accurate sizing, which has the potential to translate into improved long-term outcomes.”
“The results of the LANDMARK trial are not just a win for us but for the entire medical community and, most importantly, for patients undergoing TAVR,” added Sanjeev Bhatt, senior vice president of corporate strategy for Meril. “This study not only reinforces the safety and efficacy of the Myval THV series but also highlights its adaptability to challenging anatomical structures.”
These findings only represent the first 30 days after implant; much more research is still needed, and researchers plan to follow these patients for a total of 10 years.
The Myval valve is not yet approved by the U.S. Food and Drug Administration for use in the United States.
This 30-day LANDMARK data is scheduled to be published in The Lancet sometime in 2024.