Early outcomes with Myval TAVR valve comparable to popular devices from Medtronic, Edwards
The balloon-expandable Myval transcatheter heart valve (THV) is associated with 30-day patient outcomes that are noninferior to popular transcatheter aortic valve replacement (TAVR) valves from Medtronic and Edwards Lifesciences, according to a new LANDMARK subanalysis published in EuroIntervention.[1]
India-based Meril Life Sciences has been manufacturing its Myval heart valves for years. The devices are already approved and available in both India and Europe, though they have not yet gained U.S. Food and Drug Administration approval.
The LANDMARK trial, which is funded by Meril Life Sciences, included data from 768 low-risk patients undergoing transfemoral TAVR for severe aortic stenosis. Patients received a Myval TAVR valve, a contemporary self-expanding Evolut TAVR valve from Medtronic or a contemporary balloon-expandable Sapien TAVR valve from Edwards Lifesciences.
Initial 30-day outcomes from LANDMARK were presented at EuroPCR 2024 in Paris and then published in The Lancet.[2] That first study compared the Myval valve to a combined control group that included both the Evolut and Sapien devices; this subanalysis separated the Evolut and Sapien 3 devices into two different control groups, resulting in more detailed comparisons.
“The analytical plan and statistical design enabled us to separately test for the non-inferiority of the Myval THV series against the Sapien or Evolut THV series in a separate fashion,” wrote first author Niels van Royen, MD, PhD, a cardiologist with Radboud University Medical Center in The Netherlands, and colleagues. “Notably, this is also the first randomized comparison of two balloon-expandable valve technologies.”
While 50% of patients were treated with a Myval valve, 25% received an Evolut valve and another 25% received a Sapien valve. The mean patient ages were 80 years old for the Myval group, 79.7 years old for the Evolut group and 81.1 years old for the Sapien group. In addition, the three patient groups had similar percentages of female patients, mean body mass indexes and median Society of Thoracic Surgeons risk scores.
The study’s primary outcome was a composite of all-cause mortality, stroke, major bleeding events, acute kidney injuries, major vascular complications, moderate or severe prosthetic valve regurgitation, and conduction system disturbances leading to a new permanent pacemaker implantation (PPMI) after 30 days. These issues were seen in 24.7% of patients with a Myval valve, 30% of patients with an Evolut valve and 24.1% of patients with a Sapien valve. PPMI rates were also comparable for the three TAVR valves.
Meanwhile, the technical success rates were 96.3%, 94.7% and 98.9% for the Myval, Evolut and Sapien valves, respectively. The 30-day device success rates were 91%, 92.6% and 86.7%, respectively.
According to the study’s authors, these findings confirm that the Myval valve is noninferior to both contemporary TAVR platforms in terms of safety and effectiveness after 30 days.
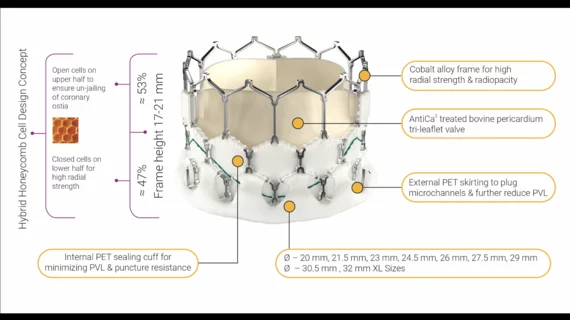
The group also examined the primary difference between the Myval valve and the contemporary offerings from Medtronic and Edwards: it is available in more size than those other platforms.
“The novel and salient feature of the Myval THV is its wide range of nominal device sizes, with 1.5 mm increments in diameter providing intermediate nominal valve diameters in addition to conventional sizes,” they wrote. “The intermediate sizes rely on manufacturing the cobalt alloy tube in the different specific diameters before laser cutting the valve frame. Diversifying device sizes enables a more precise and appropriate match between the device and the individual's aortic annular dimensions. Mismatch between the two could result in oversizing the device with respect to the individual's anatomy, resulting in aortic annular rupture or conduction disturbances, whereas undersizing could lead to paravalvular leaks, malapposition or even valve migration. Severe patient–prosthesis mismatch was 4% in both the Myval and contemporary groups.”
Researchers plan to follow these patients for up to 10 years, likely providing LANDMARK updates along the way as more data are made available.
Click here to read the full analysis.