FDA announces recall of nearly 8,000 Impella blood pumps due to risk of injury or death in TAVR patients
The U.S. Food and Drug Administration (FDA) has announced that Abiomed is recalling all of its left-sided Impella blood pumps. This is a Class I recall, which means using these devices “may cause serious injuries or death.”
However, the devices do not need to be returned; the recall is in place to make customers more aware of the issue.
Abiomed’s recall includes 7,895 blood pumps distributed from May 2021 to the present day. Exact devices impacted by the recall are the Impella 5.0 Blood Pump, Impella CP Blood Pump, Impella 2.5 Blood Pump, Impella CP with SmartAssist Blood Pump, Impella LD Blood Pump and Impella 5.5 with SmartAssist Blood Pump. Product numbers are available on the FDA’s website.
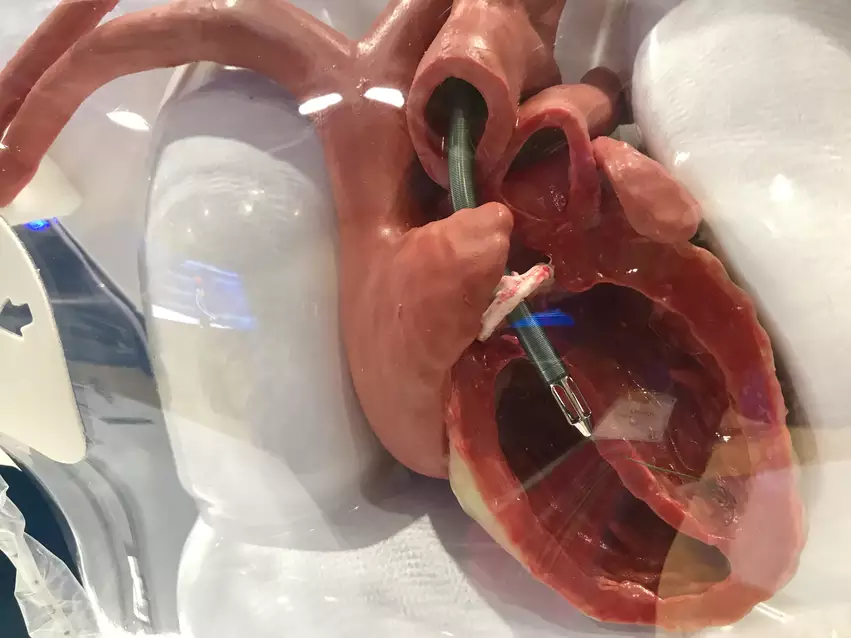
These devices are being recalled due to potential issues that can occur if they are implanted into a patient who has already undergone transcatheter aortic valve replacement (TAVR). Abiomed said the blood pumps’ instructions “do not adequately address” the precautions that should be taken when treating patients with a history of TAVR, resulting in a risk of the blood pump’s motor interacting with the TAVR valve. Such interactions could damage or even destroy the motor’s blades.
“The damaged Impella system may have reduced blood flow or pump stop, which may delay therapy or fail to provide enough support to the patient,” according to the advisory. “This could be life threatening in people who require high levels of support. There is also a risk that pieces of the broken blades could enter the patient’s bloodstream.”
There have been 30 complaints so far about this issue, including 26 injuries and four deaths.
Impella blood pumps included in the recall do not need to be returned
Abiomed emphasized that these devices are not being removed from the market and do not need to be returned. The recall is in place to ensure healthcare providers are aware of the potential risks associated with using these devices in patients who have previously undergone TAVR.
Clinicians are advised to “position the Impella system carefully” in TAVR patients, “avoid repositioning while the device is spinning” and “turn the device to P0 during repositioning or any movement that could bring the outlet windows into proximity to the valve stent structures.” In addition, if low flow is observed in any patient with a TAVR valve, it is recommended that the Impella pump be replaced “as soon as possible.”
Abiomed sent an Urgent Medical Device Correction to customers on June 14 detailing the issue. Customers are asked to review, sign and then return the response form to the appropriate recall coordinator. A copy also should be posted in a visible area to help increase awareness. Abiomed also asked customers to forward the notice to any other facilities that may need to know about the issue.
This announcement comes one month after the company recalled more than 450 Impella heart pumps due to a heightened risk of purge fluid leaking from the purge sidearm of the pump.