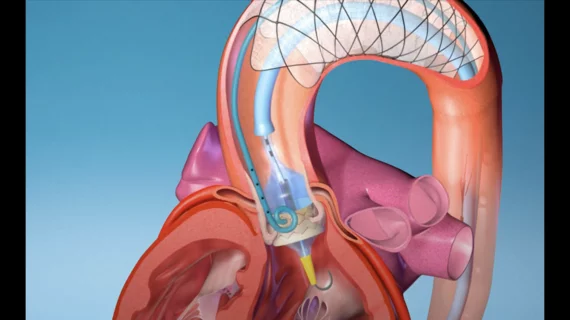
Interventional cardiologists perform world’s first TAVR with new embolic protection device
A new-look embolic protection device from Nevada-based EnCompass Technologies has been used for the very first time to help cardiologists complete transcatheter aortic valve replacement (TAVR) procedures.
A total of three TAVR procedures were performed using the F2 embolic filter device at the Israeli-Georgian Medical Research Clinic in Tbilisi, Georgia, in late April. The procedures were part of a pilot clinical trial being led by Irakli Gogorishvili, MD, PhD, head of interventional cardiology at the Israeli-Georgian Medical Research Clinic, and Tamim Nazif, MD, and Isaac George, MD, from Columbia University in New York City.
The F2 embolic filter device, like other embolic protection devices, was designed to limit the risk of stroke and other severe brain injuries during TAVR. Stroke remains one of the most significant side effects of TAVR and a primary target of researchers hoping to make the procedure safer for patients who present with severe aortic stenosis. What sets the F2 embolic filter device apart from these other solutions already on the market, according to EnCompass Technologies, is its advanced filtering capabilities and stability.
All three TAVR procedures performed using this new device were viewed as a success.
“The F2 filter was relatively easy to insert, deploy, and retrieve,” George said in a statement. “Its position remained stable throughout the procedure, despite passage of wires and large catheters. Clearly, the early results are encouraging.”
Research is scheduled to continue so that data can eventually be presented to the U.S. Food and Drug Administration and other regulators around the world.
Additional details about embolic protection devices and TAVR
The use of cerebral embolic protection devices during TAVR procedures is a hot topic among interventional cardiologists. A key late-breaking clinical trial at TCT 2022, for example, concluded that Boston Scientific’s Sentinel Cerebral Protection System was not associated with a significant reduction in the number of periprocedural strokes within 72 hours of TAVR. However, that same study—PROTECTED TAVR, which included data from 3,000 patients—did detect a connection between the use of these devices and a reduced risk of disabling stroke.
Samir Kapadia, MD, chair of the department of cardiovascular medicine at Cleveland Clinic, presented the PROTECTED TAVR findings at TCT 2022. Michael Mack, MD, the medical director of cardiothoracic surgery for Baylor Scott & White Health, was on stage as a panelist during Kapadia’s presentation. He noted that there was both a positive and a negative reading of these results—the positive was the statistics on disabling stroke, but the negative was the larger finding that the overall stroke rate was not impacted.
Kapadia discussed this subject at length with Cardiovascular Business during an exclusive video interview.