JenaValve raises $100M for its transfemoral TAVR system for severe aortic regurgitation
JenaValve Technology, the California-based medical device company focused on developing new transcatheter aortic valve replacement (TAVR) solutions, has closed a Series C financing round worth $100 million.
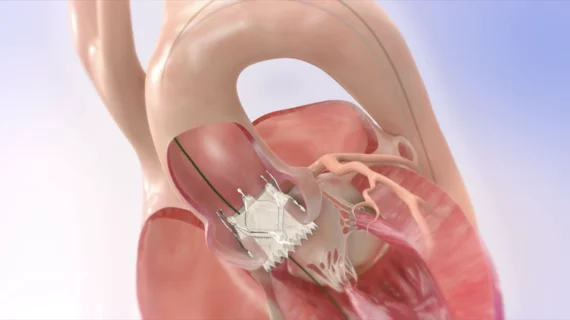
The company said it plans to use the funds to complete the research needed to gain approval from the U.S. Food and Drug Administration (FDA) for its Trilogy Heart Valve System for high-risk patients with symptomatic, severe aortic regurgitation (AR) and launch that system in the United States. The funds will also go toward improving the company’s data development and manufacturing efforts.
“JenaValve is committed to becoming the first and only FDA-approved transfemoral transcatheter valve system indicated for symptomatic, severe AR, addressing an estimated multi-billion-dollar U.S. market opportunity,” John Kilcoyne, JenaValve CEO, said in a statement. “This financing provides sufficient capital for us to conclude our ALIGN-AR clinical trial and prepare for a commercial launch in the U.S.”
The financing round was led by Bain Capital Life Sciences, but also included a mix of existing investors (Andera Partners, Valiance Advisors, Gimv, Cormorant Asset Management, RMM and Venture Incubator) and first-time investors (Pictet Alternative Advisors SA, Qatar Investment Authority, Innovatus Capital Partners and Peijia Medical Limited).
“We continue to maintain conviction in the strength of JenaValve’s differentiated technology, its prospects for entering the U.S. market, and potential for substantial long-term value creation,” added Andrew Hack, MD, PhD, Bain Capital’s managing director and a member of the JenaValve Board of Directors.
The Trilogy Heart Valve System gained CE mark approval in May 2021. In June 2022, JenaValve provided an update on the first European patients to receive the solution, highlighting their “robust clinical outcomes.”
“The early results demonstrate that our Trilogy Heart Valve System can be safe and effective for patients suffering from AR and aortic stenosis outside a clinical trial,” Kilcoyne said at the time. “These results mark another milestone in JenaValve’s journey to becoming the standard of care for the treatment of AR, as these were the first implants of their kind in the real world.”