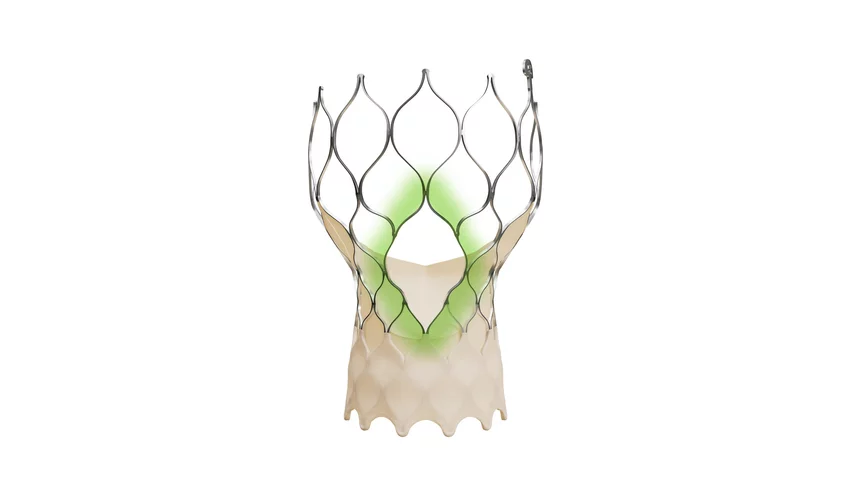
Medtronic’s next-generation TAVR device receives CE mark approval
Medtronic has received CE mark approval for its Evolut FX+ transcatheter aortic valve replacement (TAVR) system for the treatment of symptomatic severe aortic stenosis (AS). The device received U.S. Food and Drug Administration approval back in March.
Medtronic designed the Evolut FX+ TAVR system with improved coronary access in mind. Its coronary access windows are four times larger than previous Evolut valves, improving the potential for catheter maneuverability if the patient requires future coronary interventions following treatment. Interventional cardiologists are focused on the lifetime management of patients now more than ever due to the increased use of TAVR among younger patients. A considerable number of those patients could later require immediate treatment following a myocardial infarction or other adverse health event. Improving access to their coronaries could save care teams valuable time down the road.
“We are excited to complete this momentous milestone and expand our solutions for severe aortic stenosis across Europe,” Jorie Soskin, vice president and general manager of structural heart for Medtronic, said in a prepared statement. “Through our promise of continued innovation for the Evolut TAVI platform, we have delivered a more advanced and minimally invasive treatment option with proven valve performance and durability to physicians and patients across the globe.”
“The Medtronic Evolut FX+ system represents a significant step forward in the evolution of heart valve disease care,” added interventional cardiologist Didier Tchetche, MD, director of the structural heart disease department at Clinique Pasteur in Toulouse, France. “This advanced technology enables coronary access across multiple patient anatomies and offers clinicians a contemporary tool to improve outcomes without compromising an established valve performance. We are excited to see this innovative solution approved in European regions and look forward to using it for the benefit of patients.”
This approval covers AS patients from all risk categories. Medtronic expects the Evolut FX+ TAVR system to be commercially available in Europe in the weeks ahead.