New TAVR system for pure aortic regurgitation used for first time, linked to ‘excellent’ short-term outcomes
Cardiologists and cardiovascular surgeons in China have made a bit of history, using a new-look transcatheter aortic valve replacement (TAVR) system for the very first time to treat patients with pure aortic regurgitation (AR). The group detailed its findings in JACC: Cardiovascular Interventions.[1]
“In the past decade, the role of TAVR in the management of pure AR has been explored,” wrote corresponding author Yingqiang Guo, MD, with the department of cardiovascular surgery at West China Hospital, and colleagues. “However, initial reports documented high rates of residual AR and device migration, necessitating surgery or second valve implantation.”
For this first-in-human study, Guo et al. evaluated the Pioneer TAVR system from KOKA Lifesciences, a self-expanding device designed to treat AR and improve alignment. It includes three independently controllable locators intended to help TAVR operators ensure each patient’s individual anatomy captures the valve leaflet.
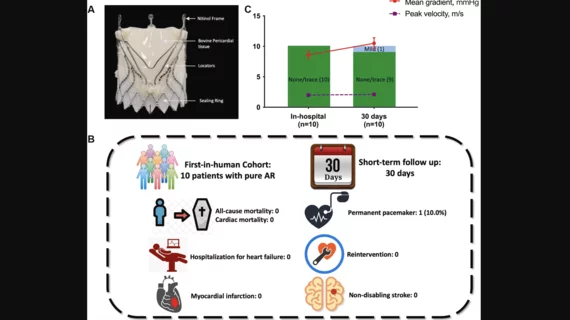
Ten patients underwent transfemoral TAVR with the new device from January to April 2023. The mean age was 72.5 years old, 60% were women and 100% presented with pure severe AR. Overall, the study’s success rate was 100%. The mean procedure duration was 42 minutes, and the mean length of stay was 8.8 days. The mean intended area oversizing was 6%.
After 30 days, the mortality rate was 0%. No patients experienced a post-TAVR myocardial infarction or stroke. One patient required a permanent pacemaker due to complete left bundle branch block, but this was the only adverse event recorded. The mean gradient was 10.5 mm Hg. One patient presented with signs of paravalvular leak after 30 days.
“The present first-in-human study suggests that the implantation of this novel TAVR system is feasible and safe,” the authors wrote. “Short-term outcomes were excellent, with no mortality, and only 1 patient received a permanent pacemaker.”
The authors emphasized that this was a small sample size and much more research will be required to confirm the device is as safe and feasible.
“A large multicenter clinical trial is currently being designed to further assess the mid-term safety and efficacy of this TAVR system,” the group concluded.
Click here to read the full analysis in JACC: Cardiovascular Interventions, a journal from the American College of Cardiology.