Self-cleaning TAVR valve offers potential of fewer clots, better outcomes
Researchers are developing a new-look prosthetic valve for transcatheter aortic valve replacement (TAVR) that could represent a significant breakthrough for patients with severe aortic stenosis (AS). The valve, still a work in progress, would continuously clean itself in a way that improves durability by limiting the formation of blood clots over time.
The team behind this project shared an early look at its work in European Heart Journal, a European Society of Cardiology journal.[1]
“Prosthetic heart valves, both biological and mechanical, have improved the survival rate and the quality of life of aortic valve heart disease patients over the last six decades,” wrote corresponding author Netanel Korin, PhD, an engineer with Technion Israel Institute of Technology, and colleagues. “While biological heart valves, taken from animal tissue and implanted via a TAVR procedure, show improved hemodynamics, their durability is limited and frequently require replacement within 10–15 years. However, mechanical heart valves, surgically implanted, can last a lifetime, but necessitate lifelong anticoagulation therapy to mitigate thrombosis risks. Thus, there is a critical need for durable prosthetic heart valves that do not require anticoagulants.”
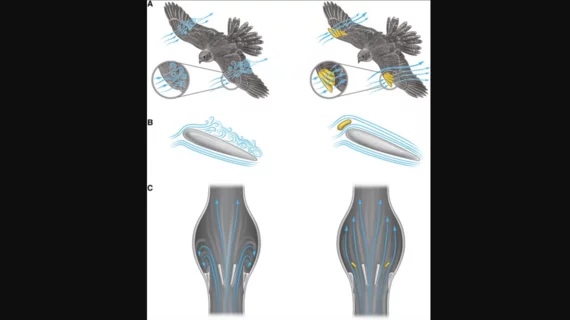
The group noted that TAVR valves “inherently produce pro-thrombotic pathological flow structures,” meaning that even advances in building materials have failed to effectively reduce the risk of flow-associated thrombosis. Their proposed solution involves designing prosthetic heart valves that “circumvent pathological flow structures”—an idea they took from the way different animals move in nature.
Passive flow control, the researchers explained, is the concept that explains how whales swim and birds fly without actively using energy. Engineers have used passive flow control when designing airplanes for many years—and now Korin et al. are hoping the same idea can be applied to heart valves.
“Although there has been some work trying to apply this general concept to improve cardiovascular devices, such as using vortex generators in mechanical heart valves, its general application in cardiovascular devices and in prosthetic heart valves so far has been very limited,” they wrote.
The research team, with financial support from the European Research Council, has used this concept of passive flow control to move forward with its new StreamlineValve Project.
“We plan to use passive flow control strategies to modulate the flow field such that it reduces primary factors contributing to coagulation on prosthetic heart valves, for instance, reducing stagnation areas, residence time, high shear stresses, and turbulence,” they explained. “The redirection of flow allows the flow-controlled prosthetic heart valve to act as a self-cleaning valve that maintains a clot-free environment around the valve. Moreover, using this approach could also lead to a homogenous pressure across all flow regions, consequently improving valve fluctuations, reducing the peak bloodstream velocity and hence reducing turbulence zones, and uniquely ensuring uninterrupted blood flow to coronary arteries through the valve’s distinctive structure.”
The researchers have found early success so far with their work, but it is still early; the ultimate goal is to improve valve durability and patient safety, they wrote, “which is particularly crucial or young patients facing lifelong implantation and the associated risks.”
Read the full breakdown of the group’s work here.