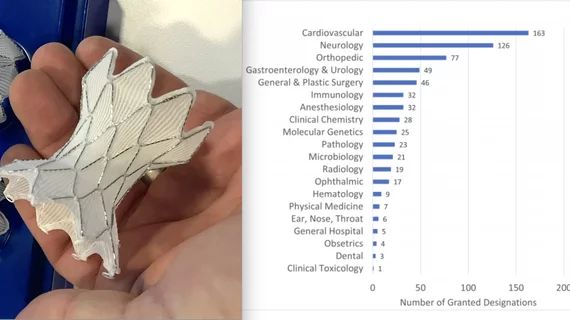
Cardiovascular devices lead FDA Breakthrough Device Designation program
U.S. Food and Drug Administration (FDA) said cardiovascular devices make up the largest number of technologies accepted into the FDA Breakthrough Devices Program. These devices also make up a large percentage of devices that have made it to final FDA market clearance. The agency this week released a list of the first 50 devices in the program that gained market clearance.
The Breakthrough Devices Program is a voluntary program for certain medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases. The goal of the program is to provide patients and healthcare providers with timely access to these medical devices by speeding up their development, assessment and regulatory review. The FDA said it speeds up the review process while preserving the statutory standards for premarket approval (PMA), 510(k) clearance, and de novo marketing authorization.
The FDA list of Breakthrough Devices that have been authorized for marketing, include 10 cardiovascular devices on the list of 50. These include:
• Shockwave Intravascular Lithotripsy (IVL) System, enabled low-pressure angioplasty balloon dilatation of severely calcified parties without vessel trauma, Shockwave Medical.
• Harmony Transcatheter Pulmonary Valve (TPV) for management of patients with severe pulmonary regurgitation, Medtronic.
• CavaClear Laser Sheath for Inferior Vena Cava Filter Retrieval, Spectranetics (Philips).
• Optimizer Smart System, pacemaker-sized impulse generator delivers cardiac contractility modulation therapyto improve 6-minute walk and quality of life in NYHA Class III heart failure patients, Impulse Dynamics.
• Caption Guidance artificial intelligence (AI) cardiac ultrasound image acquisition and optimization guidance for echocardiograms, Caption Health, Bay Labs.
• Barostim Neo System, pacemaker-sized carotid sinus nerve stimulator for improvement of heart failure symptoms, CVRx.
• Trevo Pro Vue Retriever and Trevo XP Pro Vue Retriever, neurovascular mechanical thrombectomy device for acute ischemic stroke treatment, Concentric Medical.
• Thoraflex Hybrid Stent Graft for thoracic aortic lesion treatment, Vascutek Ltd.
• TAG Thoracic Branch Endoprosthesis System endovascular graft to treat aortic aneurysm, W. L. Gore and Associates.
• OCS Heart System, expands indication for donation-after-circulatory-death donor hearts, TransMedics.
Find the complete list of breakthrough designation devices cleared by the FDA.
Cardiologists say FDA breakthrough designation has brought paradigm shift technologies into clinical practice
The Shockwave Intravascular Lithotripsy (IVL) system was cleared by the FDA in 2021 and has been called a pardigm shift in how interventional cardiologists treat calcified vessels, according to Dean Kereiakes, MD, medical director of The Christ Hospital Heart and Vascular Center in Cincinnati, and co-principal investigator of the DISRUPT CAD III FDA pivotal study. Prior to this device hard calcium in coronary vessels had to be cracked using high pressure balloons that can cause vessel damage, or cut out of using atherectomy catheters.
“The brilliance of Shockwave is that it’s a leading-edge technology packaged and delivered in a primitive device which we as interventionalists use all the time: a balloon,” Kereiakes explained. “It’s the most reliable, effective, and safe calcium-modifying technology now available, particularly in larger vessels and longer target lesions. That’s why I believe its adoption by the interventional community will be easy and swift.”
Caption Health's AI system that can guide novice sonographers to perform high quality and reproducible cardiac ultrasound exams has been used by several top hospitals in the country in trials and since FDA clearance.
“Developing AI that mimics an expert physician's eye with comparable accuracy to automatically calculate EF—including from the parasternal long-axis view, which has never been done before—is a major breakthrough,” explained cardiac ultrasound thought leader Roberto Lang, MD, a professor at the University of Chicago Medicine and past president of the American Society of Echocardiography (ASE). “Whether you are assessing cardiac function rapidly, or looking to monitor changes in EF in patients with heart failure, Caption Interpretation produces a very reliable assessment.”
CVRx Inc.’s Barostim Neo pacemaker-like system was the first to offer a way to improve heart failure symptoms in patients who did not qualify for traditional pacemakers, implantable cardioverter defibrillators (ICD), or cardiac resynchronization therapy (CRT) devices.
“Patients with advanced heart failure have limitations of physical activity, experiencing fatigue, palpitation or shortness of breath with activity and may not benefit from standard treatments, including currently marketed drugs and devices,” explained Bram Zuckerman, MD, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, when the device was cleared. “This approval provides patients with a new treatment option for the symptoms associated with advanced heart failure.”
What is the FDA Breakthrough Devices Designation program?
The FDA created the program in 2018 and a way to expedite approval of promising new technologies that appear to have promise to have a major impact in patient care. It was authorized through the 21st Century Cures Act (Cures Act).
The Breakthrough Devices Program offers manufacturers an opportunity to interact with the FDA's experts through several different program options to efficiently address topics as they arise during the premarket review phase. This can help manufacturers receive feedback from the FDA and identify areas of agreement in a timely way. Manufacturers can also expect prioritized review of their submission.
Devices are eligible for breakthrough device designation if it represents breakthrough technology, no approved alternatives exist, offers significant advantages over existing cleared alternatives and device availability is in the best interest of patients. The device also needs to provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating human disease or conditions.
Related Cardiac FDA Breakthrough Devices Content:
VIDEO: Shockwave Medical demonstrates faster intravascular lithotripsy at ACC.22
FDA approves Barostim Neo to treat HF symptoms
IVL Turns Standard Balloon Technology into a Powerful Calcium-disruption Tool
Better Together? An Integrated Market for Calcium-modification Strategies
Caption Health gains FDA clearance for AI-powered ejection fraction software
FDA greenlights AI-guided CV ultrasound acquisition system
Harmony TPV could ‘fundamentally alter’ care for patients with congenital heart disease
An industry first: FDA authorizes marketing of laser-assisted IVC filter removal device
FDA approves implantable device for CRT-ineligible HF patients