FDA grants new implant for BTK arterial disease its breakthrough device designation
Elixir Medical, a California-based healthcare technology company focused on coronary and peripheral artery disease, has received the FDA’s breakthrough device designation for a new implantable device designed to treat blocked below-the-knee blood vessels in chronic limb-threatening ischemia (CLTI) patients.
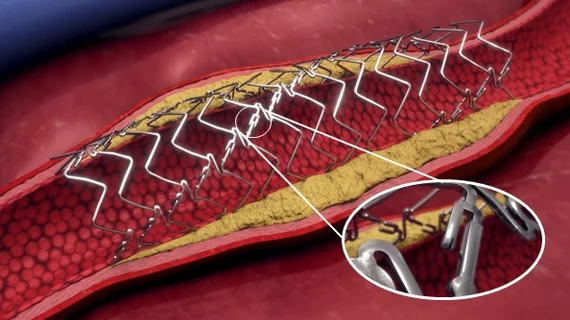
The DynamX BTK System is an adaptive device that supports a patient’s BTK vessel after PCI or other interventions. Once it is no longer needed, it unlocks and “uncages” the vessel while still providing additional support as the healing process continues.
According to Elixir Medical, the DynamX Bioadaptor restores vessel motion and function while increasing blood flow.
“The Bioadaptor platform was developed to transform treatment of coronary and peripheral artery disease,” Motasim Sirhan, CEO at Elixir Medical, said in a prepared statement. “We appreciate the FDA recognition of our innovation for treating the CLTI population with BTK disease and its potential impact on the patients suffering from vascular disease.”
This device is still not approved by the FDA to be sold and marketed in the United States. The FDA’s breakthrough devices program is designed to help medical devices make it through the approval process faster than they would otherwise. Agency representatives work closely with the manufacturer, for example, and any submissions related to the device are automatically prioritized.
CLTI a massive problem throughout U.S.
CLTI, the most severe form of peripheral artery disease (PAD), results in approximately 400 foot or leg amputations per year in the United States. Aiming to address this issue, the Association of Black Cardiologists (ABC), Society for Cardiovascular Angiography and Interventions (SCAI) and Society of Interventional Radiology (SIR) and Society of Vascular Surgery (SVS) recently launched the PAD Pulse Alliance to increase public awareness and encourage patients to seek care when necessary.
Read more about the new ABC/SCAI/SIR/SVS collaboration here and here.