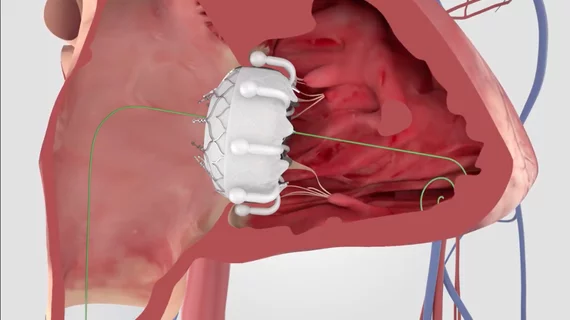
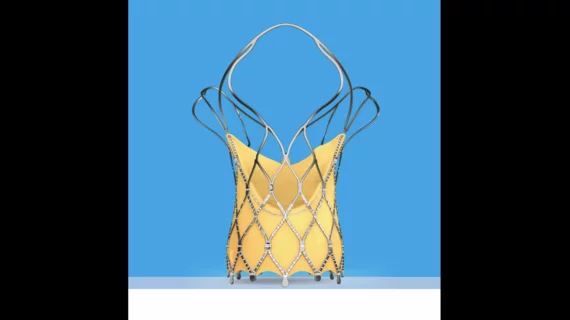
About three years ago, Betty Vaughn of Golden Valley, Minn., started to feel light-headed, fatigued and out of breath when she walked up and down the stairs. After visiting her doctor, the 89-year-old was diagnosed with degenerative mitral regurgitation (DMR), a heart condition in which the leaflets of the mitral valve do not close completely, causing blood to flow backward and leak into the left atrium of the heart. After her diagnosis, Paul Sorajja, M.D., cardiologist at Abbott Northwestern Hospital in Minneapolis, performed a transcatheter mitral valve repair (TMVR) procedure on Vaughn, who was not a good candidate for surgery, using Abbott's (NYSE: ABT) MitraClip in October 2014. Now, just months after the procedure, Vaughn has resumed many of the activities she loves, like working in the yard and playing cards with friends.