AI specialists raise $80M to ramp up commercialization of imaging analysis software
Elucid, the Boston-based healthcare technology focused on using artificial intelligence to evaluate cardiovascular disease, has raised $80 million in Series C funding. The money is expected to go toward expanding the commercialization of Elucid’s AI-powered imaging analysis software, which was designed to help physicians anticipate when major adverse cardiac events—myocardial infarctions and strokes, for example—may occur.
Elevage Medical Technologies led the funding round, with additional contributions coming from existing investors. Elucid has now raised a total of $121 million.
“This Series C round underscores the importance of Elucid’s mission to optimize the diagnosis and treatment of patients with cardiovascular disease to improve global care and outcomes,” Elucid CEO Blake Richards said in a statement.
“Elevage believes leading this investment in Elucid is pivotal to support the development and commercialization of its groundbreaking diagnostic tools,” added Elevate CEO Evan Melrose, MD. “We see an important market need for AI-powered cardiovascular software and are excited to partner with Elucid to help improve the diagnosis and management of heart disease and benefit patients worldwide.”
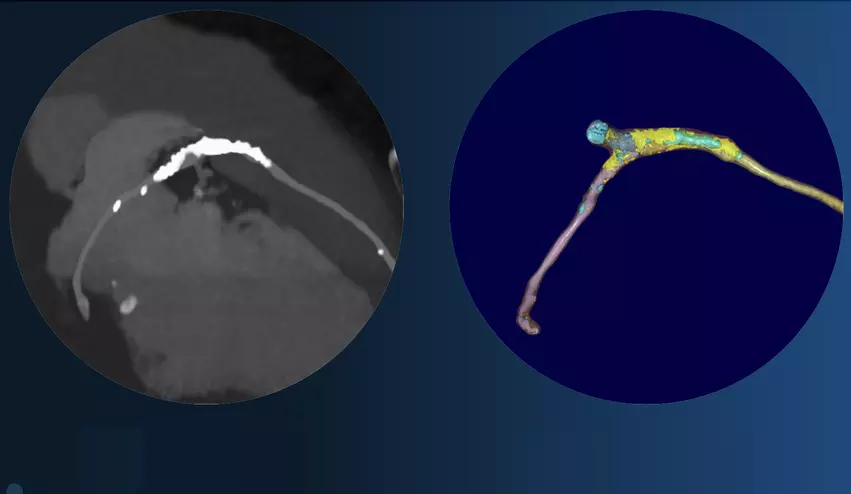
Elucid’s PlaqueIQ software includes an AI algorithm trained to read CT angiography (CTA) results and identify potential signs of cardiovascular disease. It can identify arterial plaque in CTA scans while quantifying just how significant is and helping physicians develop a treatment plan. PlaqueIQ is approved in the United States as well as Europe and South Korea. The company is also working to gain regulatory approval for an additional indication for non-invasive fractional flow reserve (FFR) assessments.
The use of cardiac CT is on the rise in the United States, thanks primarily to the 2021 chest pain guidelines developed by the American College of Cardiology and American Heart Association.
New funding round continues Elucid's busy 2023
So far 2023 has been a productive year for Elucid, with the company bringing in multiple industry veterans to bolster its executive team. In January, for example, the company named Scott Burger its chief commercial officer. Andrew Miller was named the company’s chief technology officer in March, Scott Huennekens was named its executive chairman of the board of directors in April and Windi Hary was named senior vice president of regulatory affairs and quality management in September.