Regulatory Roundup: Updates on Medtronic and LivaNova recalls, FDA-cleared AI models, a new heart valve and more
The U.S. Food and Drug Administration (FDA) has had another busy month, warning companies for selling illegal CVD supplements, approving what is expected to be the world’s most expensive drug and managing a particularly nasty influenza season.
Those news items are just the beginning, however. Here is a review of some other big FDA-related stories that have hit cardiology since the last Regulatory Roundup was published on Oct. 26:
FDA updates website to improve access to information on Medtronic’s troubled HVAD system
Medtronic’s HeartWare Ventricular Assist Device (HVAD) has been involved in multiple recalls in recent years—the issues were frequent enough, in fact, that Medtronic stopped selling and distributing the device in 2021. However, the system is still being actively used in the United States, and more problems were identified even after Medtronic stopped selling and distributing it to new customers.
In October, Medtronic announced that it had made updates available that were designed to fix problems with the pump failing to start or restart. And on Nov. 17, the FDA updated its website to gather all of the latest information on this ongoing issue and make it easier for customers to follow.
“The FDA updated this web page to enhance user access to information about safety issues with the HVAD System and recalls,” the agency wrote. “The updated information also helps patients and healthcare providers make healthcare decisions to improve health and quality of life.”
FDA clears LivaNova’s LifeSPARC system for ECMO—and the company provides a recall update
LivaNova, a London-based healthcare technology company, announced that its LifeSPARC advanced circulatory support solution has received 510(k) clearance from the FDA for extracorporeal membrane oxygenation (ECMO).
“The past couple of years have demonstrated the inherent value of the LifeSPARC system, which offers even the sickest patients a chance at survival,” Raymond Yau, MD, director of cardiogenic shock at Heart Hospital in New Mexico, said in a prepared statement.
With LifeSPARC, simplified and streamlined ECMO is in reach for healthcare centers of all sizes.”
LivaNova also provided an update on the Class I recall involving its LifeSPARC controller, emphasizing that controller updates have already been approved and cleared by the FDA that resolve the issue. Additional information on the recall from LivaNova is available here.
FDA investigating the risk of severe hypocalcemia among some kidney patients taking denosumab
The FDA has announced that it is looking into the possibility that denosumab, a prescription medicine for osteoporosis sold under the name Prolia, may be increasing the risk of severe hypocalcemia among certain patients with advanced kidney disease who are on dialysis. The agency first became aware of this potential when reviewing long-term data on patients treated with denosumab.
Hypocalcemia is a condition associated with low levels of calcium in the blood. Patients with advanced kidney disease who develop hypocalcemia could face a heightened risk of serious outcomes, including death, the FDA said in its statement.
For now, patients have been advised to keep taking the medication unless they have spoken with their physician first. Patients are also asked to alert their physician right away if they notice any signs of low calcium levels in the blood; these symptoms include tingling or numbness in the hands, painful muscle spasms or cramps, voice box/lung spasms, seizures, vomiting and irregular heartbeats.
FDA clears 2 additional advanced algorithms from Aidoc
Aidoc, one of the leading names in developing artificial intelligence (AI) models for medical imaging specialists, gained two new FDA clearances in November: one for aortic dissection and another for all vessel occlusions. Both AI models were designed to identify key findings in CT exams.
“Effective healthcare and treatment of acute conditions, like stroke and aortic dissections, require rapid, coordinated care—minutes can matter,” Aidoc CEO Elad Walach said in a prepared statement. “Our continued momentum with FDA-cleared solutions are not only aiding radiologists to prioritize potential findings, but we are helping connect other care team members immediately to the data needed to drive next actions and an optimized care plan. This leads to improved patient outcomes, such as reduced length of stay, and eases the communication burden on the care team because the right people are notified and engaged together.”
According to Aidoc, it has now acquired a total of 11 FDA clearances for its advanced AI models. The company also emphasized that it is working to expand its cardiovascular offerings.
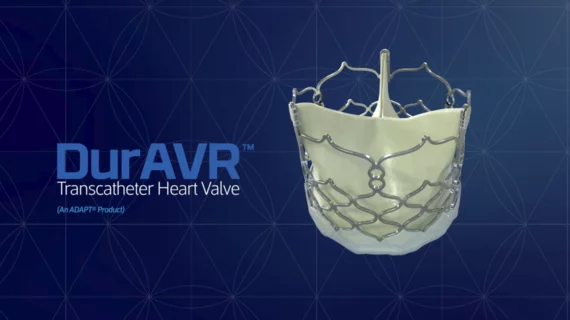
FDA approves early feasibility study for new-look heart valve from Anteris Technologies
Anteris Technologies, a structural heart technology company with offices in Australia and the United States, announced that the FDA has conditionally approved the company’s investigational device exemption application for an early feasibility study (EFS) evaluating the DurAVR transcatheter heart valve.
The EFS will enroll a total of 15 patients in the United States with symptomatic severe aortic stenosis. It is expected to begin in early 2023.
“The FDA approval to begin the DurAVR EFS is a critical milestone for Anteris achieving pre-market approval in the United States,” Anteris Technologies Chief Medical Officer Chris Meduri, MD, MPH, said in a prepared statement. “It is also another validation of the remarkable work done so far.”
“This study sets up 2023 to be a significant year of milestones and catalysts as we continue to build our remarkable base of evidence amongst patients who have had DurAVR implanted,” added Anteris Technologies CEO Wayne Paterson.
The DurAVR device is approved for investigational use in the United States as Anteris works toward gaining full FDA approval.