Regulatory Roundup: FDA clears new medical watch, considers empagliflozin for CKD and much more
The U.S. Food and Drug Administration (FDA) kicked off 2023 with a busy January. The agency approved a new transcatheter aortic valve replacement from Abbott, for example, and announced two key recalls.
Those news items, however, are just the beginning. This is a review of some other big FDA-related stories that have hit cardiology since the last Regulatory Roundup was published on Dec. 22:
1. FDA clears three different indications for CardiacSense’s medical watch
CardiacSense, an Israel-based healthcare technology company, gained FDA clearance for its CSF-3 medical watch. The agency cleared the watch for three different indications: measuring electrocardiograms (ECGs), measuring beat-by-beat heart rates and measuring oxygen saturation of arterial hemoglobin.
“Motion artifacts are the key problem plaguing other photoplethysmography-based systems,” Eldad Shemesh, CardiacSense founder and CEO, said in a prepared statement. “We have developed proprietary hardware, including a novel motion artifact sensor, which identify and cancel out motion artifacts thereby leaving us with a pure biometric signal for accurate vital sign monitoring.”
CardiacSense also has plans to submit additional data to the FDA related on the CSF-3 watch’s ability to monitor for cardiac arrhythmias and track a user’s respiratory rate and blood pressure.
The CSF-3 watch previously received CE mark approval in Europe for four indications, including the continuous monitoring of atrial fibrillation (AFib) and beat-by-beat pulse rates using photoplethysmography.
According to the statement, CardiacSense hopes to finalize terms with commercial partners in the United States and begin a U.S. launch by the end of 2023.
2. FDA grants MedAlliance its fourth investigational device exemption approval—this time for a trial studying coronary de novo lesions
MedAlliance, a Switzerland-based healthcare technology recently acquired by Cordis, received the FDA’s investigational device exemption (IDE) approval to start a new clinical trial focused on treating coronary de novo lesions with its SELUTION SLR drug-eluting balloon (DEB).
This is the company’s fourth IDE approval for a DEB so far. Enrollment on the newly-approved trial is expected to begin in the first few months of 2023.
“Treatment of de novo coronary arteries with drug-eluting balloons is a breakthrough in revascularization of coronary artery disease,” cardiologist Ron Waksman, MD, a professor of cardiology at Georgetown University, director of cardiovascular research at the MedStar Health and Vascular Institute and chairman of the MedAlliance Coronary Study Steering Committee, said in a prepared statement. “The SELUTION SLR coronary de novo study is the first of its kind in the United States and will provide important data on the efficacy and safety of sirolimus- eluting balloon as a viable alternative to drug-eluting stent, leaving nothing behind post-percutaneous coronary intervention and eliminating in-stent restenosis and related complications.”
“Coronary de novo lesions are the largest potential opportunity for use of DEB’s: the data has shown clearly that DES don’t work well in small vessels, long, or bifurcated lesions or in patients with diabetes or risk of high bleeding complications,” added Jeffrey B. Jump, chairman and CEO of MedAlliance.
The SELUTION SLR DEB has already gained CE mark approval for the treatment of peripheral artery disease and the treatment of coronary artery disease.
3. FDA approves Lupin’s abbreviated new drug application for generic version of prasugrel tablets
Lupin, an international pharmaceutical company based out of Mumbai, India, gained FDA approval for its generic version of prasugrel, which is sold under the brand name of Effient.
Prasugrel is a popular medication for helping certain cardiac patients lower their risk of suffering a myocardial infarction or stroke. The tablets will be made available in two doses: 5 mg and 10 mg.
Lupin has been focused on the treatment of cardiac conditions in recent weeks. Not long after its prasugrel announcement, the company officially launched its version of combination sacubitril and valsartan in India. The brand names of these newly launched medications are Valentas and Arnipin.
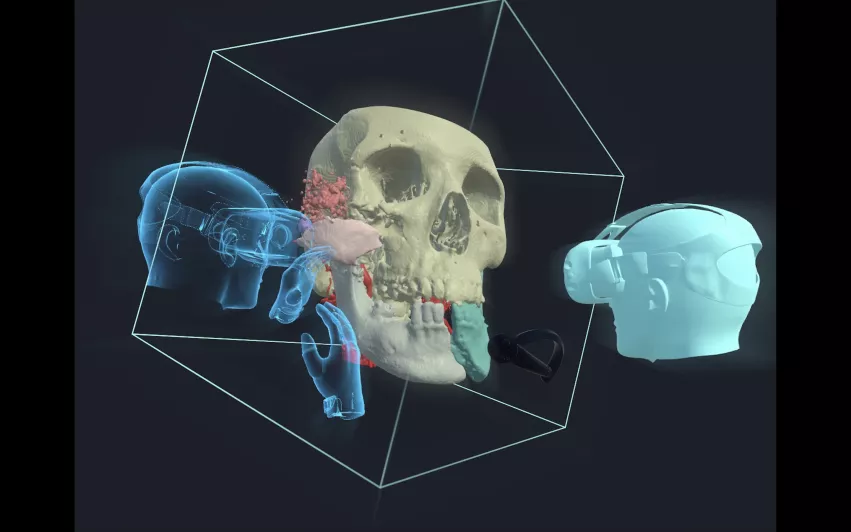
4. FDA clears Realize Medical’s VR-based surgical planning software solution
Realize Medical, a Canadian startup focused on virtual reality (VR) technology, gained FDA clearance for Elucis, the company’s VR-based surgical planning software.
With Elucis, users can view, share and interact with complex images taken from cardiac imaging exams. The software creates a 3D environment where users can collaborate with one another, and it was also designed to provide more clarity to patient consultations.
“Virtual planning with Elucis allows me to conceptualize innovative procedures with the end product of successful cases, development of new devices and improved education for patients and families,” interventional cardiologist Jenny Zablah, MD, a specialist out of Children’s Hospital Colorado, said in a prepared statement.
5. FDA accepts Eli Lilly and Company’s supplemental new drug application for empagliflozin
Eli Lilly and Company’s supplemental new drug application for empagliflozin to be a potential treatment for chronic kidney disease (CKD) has been accepted by the FDA.
Empagliflozin, sold and marketed under the name Jardiance, was originally approved by the FDA as a way to lower blood sugar in adult patients with type 2 diabetes and known cardiovascular disease. It has also previously been approved to treat patients with heart failure.
“This marks another exciting milestone for Jardiance, potentially extending its ability to positively impact the approximately one billion people diagnosed with a cardio, renal or metabolic condition,” Jeff Emmick, MD, PhD, vice president of product development for Eli Lilly and Company, said in a prepared statement. “We look forward to working with the FDA during the review process and eagerly await a decision later this year on the indication for CKD, which doubles a person's risk for hospitalization.”