Pediatric cardiology device competition introduces world to new technologies
Six medical technology innovators focused on pediatric cardiology were awarded grants of $50,000 each in the “Make Your Medical Device Pitch for Kids!” competition in Toronto on Oct. 14. The $300,000 in awards was partly funded by the U.S. Food and Drug Administration (FDA) and will help startup companies bring their new devices to market.
The Centers for Disease Control and Prevention says approximately 40,000 children are born annually with a congenital heart defect. Children with heart conditions need medical devices tailored to their specific physiological needs, since most devices currently available are sized for much larger adult patients. There is a significant unmet need for pediatric devices designed to monitor and treat pediatric patients effectively in cardiology, interventional cardiology, cardiac surgery and electrophysiology. This competitive grant program is designed to identify and support the development and commercialization of devices addressing these needs.
The competition is presented by the Alliance for Pediatric Device Innovation (APDI), a nonprofit consortium led by Children’s National Hospital. It is funded through the FDA and Additional Ventures, a nonprofit focused on accelerating research progress and improving clinical care for individuals born with single ventricle heart defects. Awardees also gain access to support services and technical expertise provided by APDI and Additional Ventures in areas that include engineering, regulatory, reimbursement, clinical trials study design and data science services.
The following six companies won out of 10 finalists in the competition:
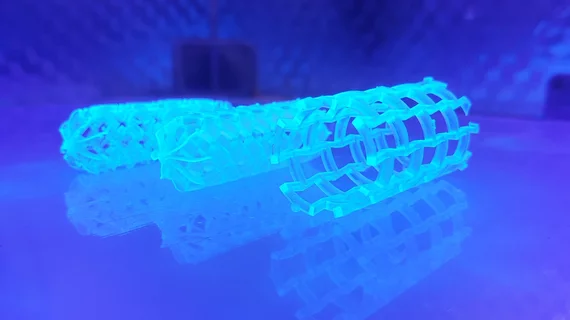
• Massachusetts Institute of Technology (Cambridge, Massachusetts) won for its polymeric auxetic stent to treat pediatric aortic coarctation. This is a 3D-printed stent that can be sized to a specific patient. The design allows growth in children with aortic coarctation when it is reabsorbed after fulfilling its function and reduces the need for frequent reinterventions.
• Bloom Standard (Minneapolis, Minnesota) won for its autonomous hands-free ultrasound. The vast majority of children lack access to pediatric ultrasound imaging in their local medical settings, and the delayed detection of respiratory and cardiac conditions is a significant issue. Most existing ultrasound solutions are expensive, immobile and can only be used in higher-level medical settings by skilled sonographers. Bloom Standard's offering can be used by anyone, anywhere to detect heart or lung abnormalities using AI-driven onboard image interpretation.
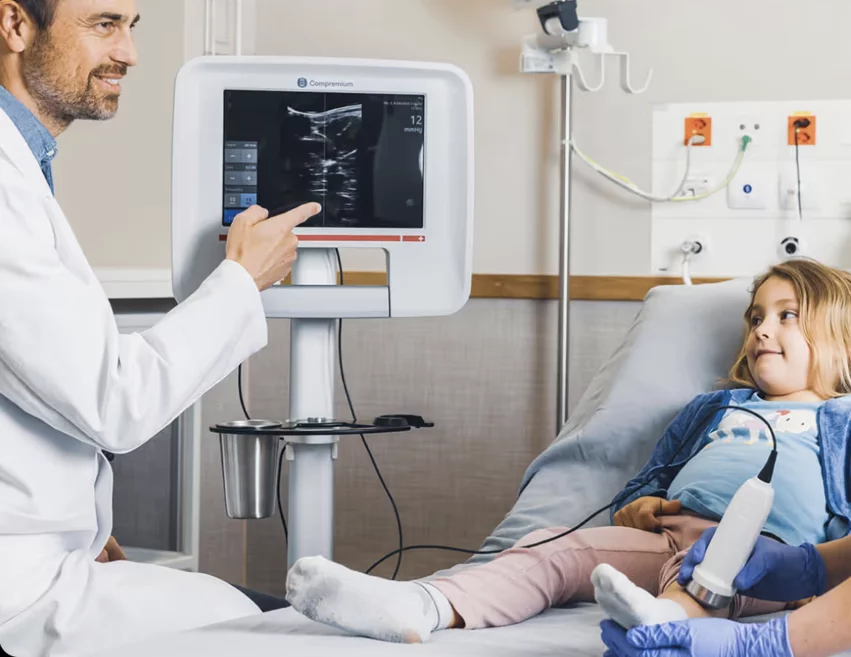
• Compremium AG (Bern, Switzerland) won for its noninvasive central venous pressure estimation system for pediatric patients. The company developed a noninvasive external assessment does away with needles for the diagnosis of venous pressure conditions. It uses AI-enabled, highly reliable and reproducible intermittent monitoring to make predictive diagnosis possible. It offers real-time compressibility measurement in less than 30 seconds.
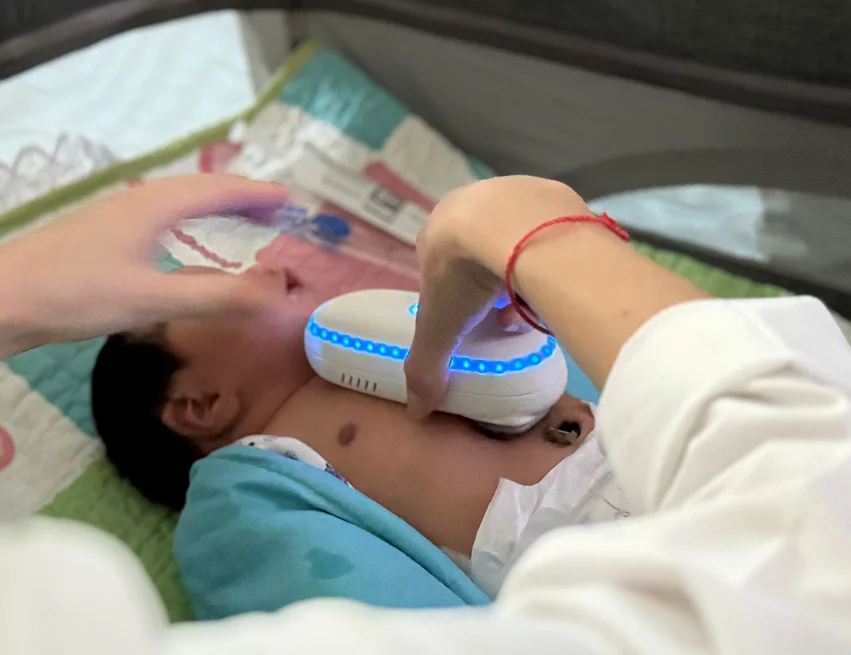
• OxiWear (Arlington, Virginia) won for its home measurement of oxygen levels in pediatric congenital heart disease patients. This is the first FDA-cleared medical wearable for measuring continuous blood oxygen levels using a wearable ear clip while a patient is in motion. The company now plans to make a pediatric version of the device to monitor pediatric patients for hypoxia.
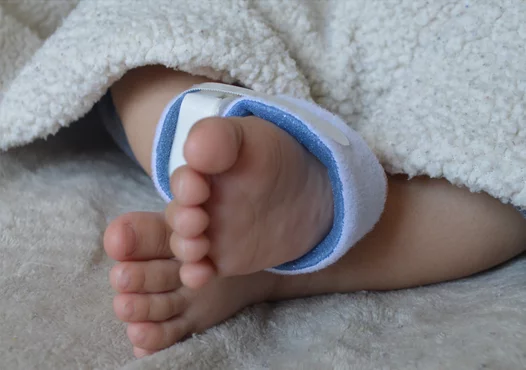
• PyrAmes Inc. (Cupertino, California) won for its wearable, noninvasive pediatric blood pressure monitor. The Boppli device is a single-use, continuous, noninvasive blood pressure monitor (cNIBP). It is placed on the arm or foot of an infant to obtain a pulse waveform signal and data is streamed to a bedside device to provide real-time information including mean arterial pressure, systolic blood pressure, diastolic blood pressure and pulse rate.
• Sibel Health (Chicago, Illinois) won for its hospital-to-home wearable monitoring for pediatric heart conditions. The Anne Limb wearable adhesive sensor is a clinical-grade monitoring device that measures SpO2, pulse rate and skin temperature. The rechargeable device seamlessly transmits real-time data for constant pediatric monitoring.
"Congratulations to our awardees, whose innovative technologies show great promise in advancing care for pediatric heart patients," said Kolaleh Eskandanian, PhD, MBA, vice president and chief innovation officer at Children's National and APDI program director and principal investigator, in a statement. "We are thrilled to welcome this new cohort into our pediatric device accelerator, where they will have the opportunity to collaborate with clinician-scientists at Children's National and connect to Additional Ventures’ network. Along with these collaborations, the awardees will benefit from a full range of APDI wraparound services designed to support the development of devices specifically for pediatric patients, helping them navigate the complex path to market."
The competition was held in conjunction with the 12th Annual Symposium on Pediatric Device Innovation, presented by Children’s National and co-located with The MedTech Conference.
“Additional Ventures is thrilled to support this new class of innovators whose products will make a profound impact in the management and care of pediatric heart patients,” Additional Ventures CEO Kristie Keller, PhD, said in a statement. “We hope that this competition both inspires and activates the community and brings much-needed new entrants and new ideas to pediatric-first device development.”