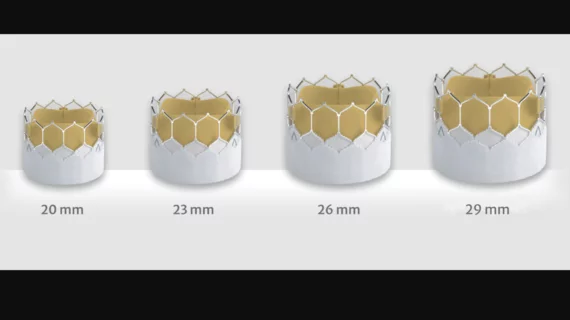
EuroPCR
PCR is a European organization dedicated to education and information in the field of interventional cardiology and minimally invasive transcatheter procedures. Its activities cover a large spectrum, from the organization of annual courses in Europe, Asia, the Middle East and Africa, to editing a scientific journal, publishing textbooks as well as providing training seminars on thematic subjects. For more information, visit https://www.pcronline.com/Courses/EuroPCR.
Displaying 49 - 56 of 64
![The use of intravascular lithotripsy (IVL) during percutaneous coronary intervention (PCI) is still safe and effective when patients present with calcified nodules (CNs), according to new long-term data published in EuroIntervention.[1] Researchers compared outcomes from patients with and without CNs, highlighting key similarities in stent expansion and luminal gain.](/sites/default/files/styles/top_stories/public/2024-12/screenshot_2024-12-02_at_11.07.21_am.png.webp?itok=YDi6SyF_)