Regulatory Roundup: FDA approves new-look self-expanding stent, clears 2 advanced AI models
The U.S. Food and Drug Administration (FDA) has had a busy month, overseeing the recall of nearly 88,000 implantable cardiac devices, juggling the continued rise of monkeypox cases in the United States and maintaining an active Breakthrough Devices program.
Here is a review of some of the biggest FDA-related stories to hit the cardiology since the last Regulatory Roundup was published July 25:
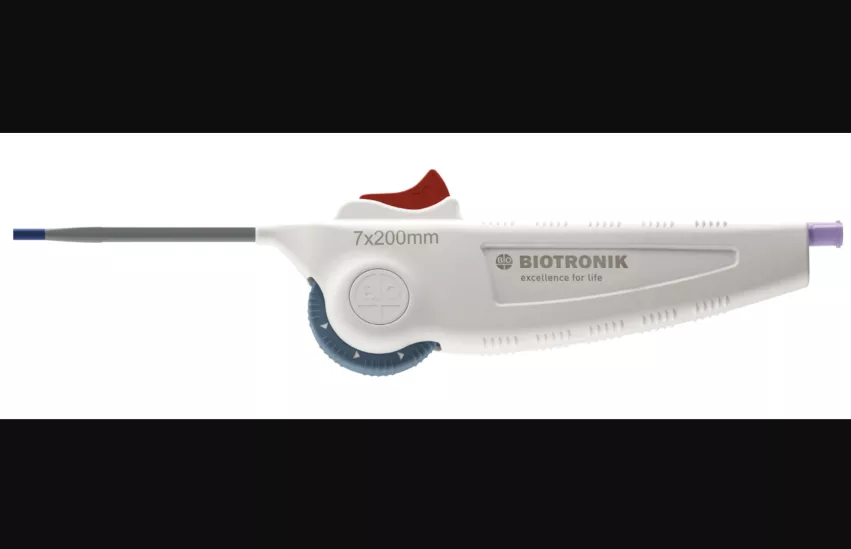
1. Biotronik gains FDA approval for a new peripheral self-expanding stent system
Biotronik, the international healthcare technology company focused on cardiovascular and endovascular health, received FDA approval for its Pulsar-18 T3 peripheral self-expanding stent system. The new offering, which includes a 4-French low-profile delivery system, a wheel-operated handle and a tri-axial system for improved implantation, is already hitting the U.S. market.
The Pulsar-18 T3 stent system is indicated for patients symptomatic de novo, restenotic or occlusive lesions in their superficial femoral or proximal popliteal arteries. It will be offered in up to a 200 mm stent length.
“The Pulsar-18 T3 stent system is an innovative solution that delivers clinically proven performance—providing effective therapy that is easy to use for physicians while minimizing metal burden and may reduce the risk of restenosis for patients,” David Hayes, MD, Biotronik’s chief medical officer, said in a statement.
2. Viz.ai gains FDA clearance for a new AI-powered subdural hemorrhage application
Viz.ai has received FDA clearance for its Viz Subdural (Viz SDH) algorithm, which the company trained to automatically detect signs of subdural hemorrhage.
The San Francisco-based artificial intelligence (AI) veterans noted that this new algorithm identifies both acute and chronic subdural bleeds, sending urgent alerts to the patient’s care team when necessary. It is now an official part of the Viz Platform.
The company also highlighted recent research that found the AI model has a sensitivity of 94% and specificity of 92%.
“Subdural hemorrhages are growing in commonality, but can present different levels of urgency with different clinical pathways,” Jayme Strauss, chief clinical officer of Viz.ai, said in a statement. “Viz SDH supports physicians by detecting the presence of subdural hemorrhage and expediting communications and image sharing to improve the clinician workflow and more efficiently and effectively treat patients experiencing subdural hemorrhages.”
Viz.ai raised $71 million in 2021 to help expand its AI offerings in the cardiovascular market.
3. Dyad Medical gains FDA clearance for a new AI-powered echocardiogram application
Dyad Medical, a Boston-based healthcare technology company focused on using artificial intelligence (AI) to assess cardiac imaging exams, received FDA clearance for Echo:Prio, its new echocardiogram evaluation application.
Echo:Prio, the latest addition to Dyad Medical’s cloud-based Libby platform, was designed to automatically assess echocardiograms, providing an immediate second opinion. Users can access the platform on a variety of devices.
“The current process of manual echocardiographic assessment of myocardial function is time consuming and lacks consistency between readings,” Ronny Shalev, PhD, Dyad Medical’s co-founder and CEO, said in a statement. “The FDA’s clearance of Dyad Medical’s Echo:Prio application allows us to provide operators and physicians an essential computer-assisted tool for echocardiographic analysis. Our solution is proven to be consistent and can be used across all systems, regardless of an operator’s skill level.”