Regulatory Roundup: FDA clears AI-powered Apple Watch competitor, grants breakthrough designation to new heart failure device
The U.S. Food and Drug Administration (FDA) has had a busy in July, approving Eko’s latest artificial intelligence (AI) algorithm for detecting heart murmurs and allowing Bayer to import a foreign-labeled contrast media agent.
Here is a review for some of the agency’s other big cardiology-related moves from the last few weeks:
1. Ra Medical Systems receives FDA clearance for its DABRA 2.0 catheter
Ra Medical Systems, the California-based healthcare technology company focused on vascular disease, gained FDA clearance for its DABRA 2.0 catheter. The updated solution, part of Ra Medical’s DABRA Excimer Laser System, was designed with improved deliverability and navigation in mind.
In a statement announcing the news, Ra Medical Systems CEO Will McGuire emphasized that the company is still evaluating how to move forward after letting go approximately 65% of its full-time employees. The remaining employees were offered “conditional retention arrangements for a period of approximately 60 days.”
“As we have previously announced, the DABRA 2.0 catheter represents an interim step in our work to develop a guidewire-compatible version of the DABRA catheter, and at this time we have no plans to commercialize the DABRA 2.0,” McGuire said.
2. iRhythm receives FDA clearance for its ZEUS System
iRhythm also announced a key FDA approval in July. The company gained FDA clearance for its ZEUS (Zio ECG Utilization Software) System, a new AI algorithm designed to help patients wearing the Zio Watch monitor their hearts for any signs of atrial fibrillation (AFib).
“We are incredibly excited about this important milestone as we make progress in bringing a new monitoring platform to patients who can benefit from it,” Quentin Blackford, CEO and president of iRhythm, said in a statement. “There is a clear need in the market today for a clinical grade, long-term and noninvasive monitoring solution.”
iRhythm highlighted the importance of its data, including what it describes as the “world’s largest repository of labeled electrocardiography (ECG) data,” to the success of its ZEUS algorithm.
iRhythm’s Zio Watch, a collaboration with Verily, will be complimentary to patients already using its Zio monitoring solutions. The noninvasive wearable is not yet commercially available, though a limited launch is expected in 2023. The new-look cardiac watch, once launched in the U.S. market, is seen as a potential competitor for the Apple Watch.
“The industry is ripe for a clinical grade wearable to not only improve how we monitor cardiovascular health, but also develop precision health interventions that could ultimately prevent more serious cardiac events before they can occur,” Jessica Mega, MD, Verily co-founder and chief medical and science officer, said in the same statement.
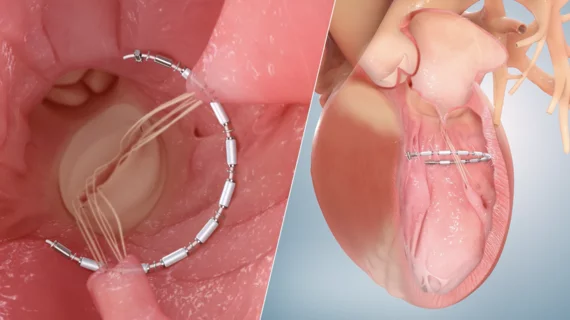
3. Ancora Heart receives FDA’s breakthrough device designation for its AccuCinch Ventricular Restoration System
Ancora Heart received the FDA’s breakthrough device designation for its AccuCinch Ventricular Restoration System, a new minimally invasive transcatheter device designed for the treatment of heart failure. This designation from the agency helps speed up the approval process.
The AccuCinch device is a flexible solution that is implanted into a patient’s left ventricle and then clinched, reducing the size of that ventricle and limiting ventricular wall stress. A clinical trial, the CORCINCH-HF study, is already underway.
Cleveland Clinic, regularly voted one of the country’s elite heart hospitals, recently categorized the AccuCinch device as one of the most promising interventional heart failure therapies on the market.
An update on cardiology-related recalls
July has been a fairly quiet month for cardiology-related recalls, a considerable change of pace compared to the first six months of the year. Medtronic did still recall more than 1 million dialysis catheters, however, a sign that even quiet months for healthcare recalls are going to include some significant news items.