FDA clears first ablation catheter with flexible electrode tip, contact force sensing
The U.S. Food and Drug Administration (FDA) recently cleared Abbott's TactiFlex Ablation Catheter, Sensor Enabled, which is the the world's first ablation catheter with a flexible tip and contact force technology. The radiofrequency (RF) catheter has been approved to treat atrial fibrillation (AFib).
This is one of many new electrophysiology (EP) technologies that have been introduced in recent years to improve catheter ablation efficacy rates in AFib. Until just a few years ago, about one in three patients had to return for repeat ablation procedures. These newer-generation technologies are also helping reduce ablation procedure times.
"We are entering the next chapter of AFib ablation with new tools such as Abbott's TactiFlex that, when used with mapping systems to accurately identify the source of an arrhythmia, can safely and efficiently treat the problem in ways we never thought possible a decade ago," Larry Chinitz, MD, director of the Heart Rhythm Center and co-director of NYU Langone Heart in New York City, said in a statement back in May.
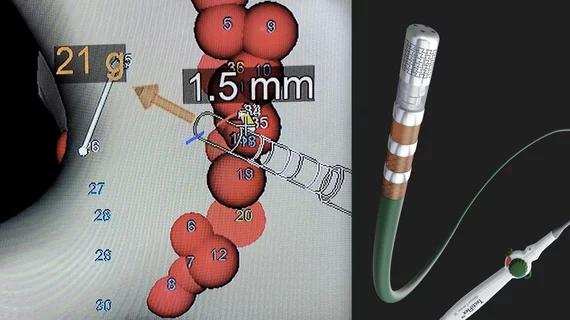
Abbott said the TactiFlex catheter can result in reduced procedure times and better safety when compared to the company's previous generation non-flexible catheter.[1] The new catheter is designed to be used with Abbott's EnSite X EP System mapping system, which allows physicians to identify areas in the heart require ablation. The integration with this system also displays the the distances between ablation lesions and the contact force of the catheter against the myocardium.
Contact force-sensing catheters were developed to help overcome the issue of incomplete ablation lesions. These poor-quality lesions allow errant electrical signals from cardiac tissue responsible for causing AFib to continue causing issues. Poor-quality lesions are often caused when the catheter does not have enough contact with the tissue for proper transfer of RF energy. By showing the electrophysiologist the pressure being applied on the catheter, it is intended to help ensure better quality lesions and reduce repeat procedures.
This catheter also uses a laser-cut pattern tip design that flexes when in contact with the heart wall. This helps enable more accurate positioning of the catheter by providing more stability as the heart constantly beats. The catheter tip can flex as the heart moves.
The TactiFlex catheter generated strong clinical outcomes in the TactiFlex AF IDE study.[2,3] The study showed the catheter created fast, safe lesions to treat AFib with over 99% acute procedural success and reduced arrhythmia recurrence.
The TactiFlex catheter is also approved for use in Europe earlier this year. It is also approved in Japan, Africa and Australia.