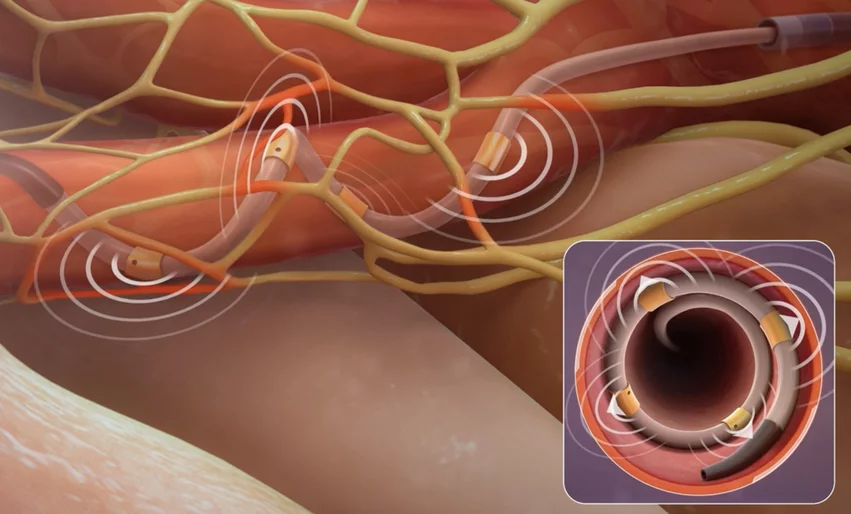
FDA approves another renal denervation system for hypertension
The U.S. Food and Drug Administration (FDA) has approved Medtronic’s Symplicity Spyral renal denervation (RDN) system for hypertension. This is the second RDN system to ever gain FDA approval for the treatment of hypertension. Recor Medical’s Paradise Ultrasound RDN system received the greenlight in early November.
Medtronic’s Symplicity Spyral RDN system treats hypertension through the use of four radiofrequency ablation electrodes attached to a single catheter. The FDA reviewed data from multiple clinical trials, including SPYRAL HTN-OFF MED and SPYRAL HTN-ON MED, when making its decision.
“Medtronic has always believed in the potential of this therapy,” Jason Weidman, senior vice president and president of Medtronic’s coronary and renal denervation business, said in a statement. “We partnered closely with leading experts in our clinical community who could help us in our journey to get this technology to the people who need it most. It was the promise of this therapy that enabled Medtronic to keep going, even when others exited the renal denervation space. High blood pressure is a global health issue, and patients need more options to manage their blood pressure.”
Medtronic has already gained CE mark approval to sell and market the Symplicity Spyral RDN system in Europe. With FDA approval acquired, the company expects to move forward with commercialization in the United States right away.
Approval comes 3 months after FDA panel voted not to endorse the Symplicity Spyral RDN system
This approval came as a bit of a surprise. An FDA advisory panel voted not to endorse the Symplicity Spyral RDN system in August. The panel voted 13 to 0 that the device was safe to use for patients with uncontrolled hypertension, and it voted 7 to 6 that the device was effective for treating the intended patient population. However, the group could not agree that the RDN system’s benefits outweigh any potential risks.
Julia B. Lewis, MD, a professor of medicine with Vanderbilt Health, was one of the panel members who had concerns about the Symplicity Spyral system’s ability to improve outcomes. She voted that its benefits did not outweigh its risks.
“I think we have to be cautious not to confound the desperateness of the unmet need with a willingness to throw anything at that unmet need,” she said at the time. “It’s not going to help people’s blood pressure and their cardiovascular outcomes to have a procedure that is potentially either ineffective or minimally effective.”
Patient selection for RDN treatment
Patient selection is seen as crucial step for any RDN system, whether it is the Symplicity Spyral or Paradise Ultrasound device. It is recommended that patients with high blood pressure start off taking medications and adapting healthier lifestyle choices. If those initial options do not produce results, it may be time for a patient’s care team to consider RDN.
"I don't think we want to get into a clinical scenario where anybody with high blood pressure is sent for denervation,” Ajay J. Kirtane, MD, a professor of medicine at NewYork-Presbyterian/Columbia University Irving Medical Center, told Cardiovascular Business in an exclusive interview published in February. “That is not the right way to approach this.”
In the same interview, Kirtane emphasized that RDN is not a flat-out cure for hypertension. Instead, it is a way to help patients who are not responding to other interventions experience improved symptoms and a better quality of life.
Interventional cardiologists highlight ‘groundbreaking' FDA approvals
The Society for Cardiovascular Angiography and Interventions (SCAI) issued a statement about Recor Medical and Medtronic both gaining FDA approval for their RDN offerings, calling the development a “groundbreaking advancement in the treatment of patients with uncontrolled hypertension.”
“The approval of the renal denervation systems by the FDA is a game-changer for both interventional cardiology and the treatment of hypertension” SCAI President George Dangas, MD, PhD, said in the statement. “This innovative technology has the potential to revolutionize how we approach the management of high blood pressure which has grown tremendously globally, offering patients a safe and effective treatment option.”
SCAI also issued a position paper about RDN in August. Click here for more information.