FDA grants new resorbable scaffold for CLTI its breakthrough device designation
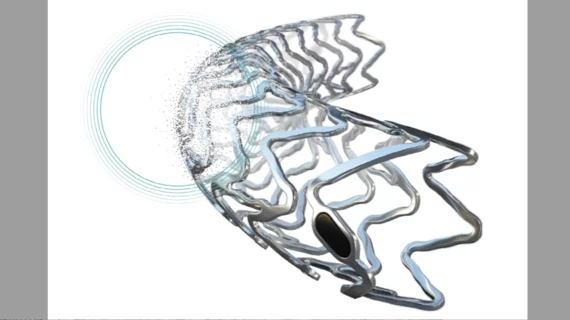
Biotronik has received the FDA’s breakthrough device designation for its Freesolve below-the-knee resorbable magnesium scaffold (BTK RMS) for patients with chronic limb-threatening ischemia (CLTI).
The company’s Freesolve technology, which gained CE mark approval in February, was designed to maximize blood flow and minimize the post-implantation risks of stent thrombosis and target lesion revascularization. It includes thinner struts than previous generations and “new markers for increased radiopacity.” More than 99% of the magnesium scaffold is resorbed after one year.
“Biotronik’s focus on vascular interventional excellence is evident in our strategic investments and persistent dedication to innovation”, said Jörg Pochert, PhD, president of vascular intervention at Biotronik, said in a statement announcing the news. “Our efforts to expand therapeutic possibilities, underlined by the introduction of the Freesolve RMS for coronary artery disease treatment, will continue in the BTK indication with this groundbreaking innovation.”
The FDA’s breakthrough devices program is designed to help medical devices make it through the approval process faster than they would otherwise. The agency’s representatives work directly with the device manufacturer, for example, and any submissions related to the device will be prioritized. This device is still not approved by the FDA to be sold and marketed in the United States.
“This breakthrough device designation for the Freesolve RMS for BTK treatment is a significant milestone in advancing treatment options … Our next generation RMS represents a leap forward over existing resorbable technology, incorporating technical innovations intended to address physicians' needs and optimize outcomes for patients suffering from CLTI,” Ryan Walters, U.S. president of Biotronik, said in the same statement.
CLTI a massive problem in the United States
CLTI is the most severe form of peripheral artery disease (PAD), which results in approximately 400 foot or leg amputations per year in the United States. Hoping to address this issue, the Association of Black Cardiologists (ABC), Society for Cardiovascular Angiography and Interventions (SCAI) and Society of Interventional Radiology (SIR) and Society of Vascular Surgery (SVS) launched a new program known as the PAD Pulse Alliance to increase public awareness and encourage patients to seek care when necessary.
Read more about the new ABC/SCAI/SIR/SVS collaboration here.