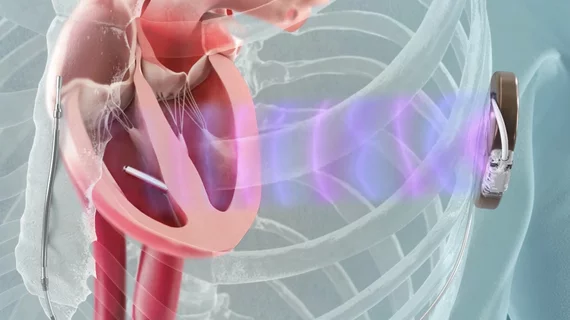
Completely leadless cardiac rhythm management system impresses
The first modular, leadless cardiac rhythm management (CRM) system composed of a leadless pacemaker and a subcutaneous, leadless implantable cardioverter defibrillator (ICD) was linked to positive early results at ESC Congress 2024 in London.
The MODULAR ATP study demonstrated a high rate of successful leadless pacemaker implantations with few complications and stable pacing parameters. The system uses the Emblem Subcutaneous Implantable Defibrillator (S-ICD) System and new Empower Leadless Pacemaker from Boston Scientific. The devices work together wirelessly to coordinate intracardiac anti-tachycardia pacing (ATP) therapy, provide rate-responsive bradycardia pacing support and prevent sudden cardiac death without the use of leads implanted inside the heart and connecting veins.
“We saw excellent overall clinical performance of the Empower leadless pacemaker in the MODULAR ATP trial, including a high rate of successful implants, few complications and stable pacing parameters,” principal investigator Lluis Mont, MD, PhD, atrial fibrillation unit head with the Hospital Clinic at the University of Barcelona, said in a statement. “These findings indicate that the device can function as a standalone pacemaker to provide rate-responsive bradycardia pacing to patients, and complement data previously published from this study, which showed a high percentage of pain-free termination of spontaneous tachyarrhythmia episodes when used in connection with the Emblem S-ICD.”
The presentation focused on data from 293 patients enrolled in the study as of Jan. 24, 2024, and evaluated the Empower's performance in rate-adaptive pacing and pacing in the ambulatory setting. The rate-response sub-study (n=35) measured the leadless pacemaker’s accelerometer response to support rate-adaptive pacing. Another 31 patients underwent Holter monitoring to confirm ambulatory performance.
The study showed pacing performance measures were stable and within acceptable ranges. Accelerometer performance showed a mean MCR slope of 0.96, (95% confidence limits 0.91-1.02). The Holter sub-study showed appropriate pacing behavior. At six months, 97% of patients had a pacing burden ≤10%.
The MODULAR ATP study was leached in December 2021. Boston Scientific said it plans to pursue FDA approval for the Empower leadless pacemaker and the CRM system in 2025.
Leadless CRM could have a significant impact on patient care
Modular CMR therapy could represent a paradigm shift in the device technologies used in the future. There has been a movement toward using leadless pacemakers, especially over the past year since the FDA clearance of a dual-chamber leadless device. These devices simplify delivery of the pacemaker via a catheter procedure and reduce the complications associated with open surgical procedures to implant the device, especially in terms on infection rates. Leadless devices also help with future management of these patients by avoiding the need for additional open procedures and eliminating issues associated with lead management and removal of old leads.
The S-ICD was seen as a revolutionary advancement when first introduced in 2012. It eliminated the use of leads inside veins and inside the heart, instead using a minimally invasive port surgical approach for a subcutaneous lead on the sternum. The device has been implanted in more than 100,000 patients worldwide.