FDA clears new software for AI-powered CCTA assessments
Elucid, a Boston-based healthcare technology focused on using artificial intelligence to identify signs of cardiovascular disease (CVD), has received U.S. Food and Drug Administration (FDA) clearance for its PlaqueIQ imaging analysis software.
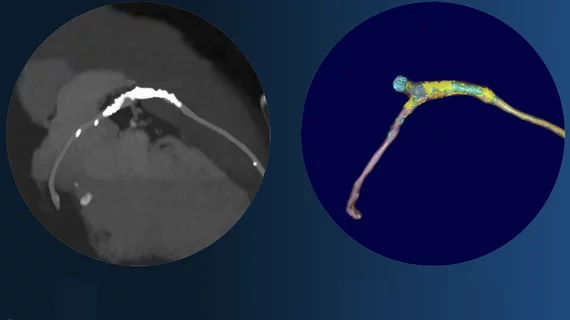
PlaqueIQ was trained to turn coronary CT angiography (CCTA) images into interactive 3D reports that help physicians visualize the presence of atherosclerosis. It also classifies and quantifies tissue structure and composition.
The cloud-based software represents the latest version of Elucid’s PlaqueIQ technology. Previous versions had gained FDA clearance for similar indications, but they lacked the commercial features that are now included.
“Elucid’s mission is to commercialize proven technologies that can make a meaningful difference in the prevalence of heart attack and stroke, and the FDA clearance of PlaqueIQ is a huge step forward towards that goal,” Kelly Huang, CEO of Elucid, said in a statement. “We know that plaque is the key contributor to these devastating events, and, specifically, high-risk plaque components, but you can’t treat what you can’t see.”
“It’s time to shift our focus from merely estimating risk and treating risk of myocardial infarction to directly visualizing and treating the disease itself by looking at the coronary arteries,” added Amir Ahmadi, MD, a cardiac imaging specialist with Icahn School of Medicine at Mount Sinai and co-director of the cardiac intensive care unit at Mount Sinai Fuster Heart Hospital at Morningside. “I believe that PlaqueIQ will enable physicians to better ‘see’ the disease—specifically plaque quantity and type—so that we can treat patients with greater precision and in personalized manner, improve their quality of life, and ultimately prevent MI and stroke more effectively.”
PlaqueIQ is already in the hands of U.S. clinicians for beta testing. Elucid plans on making the AI-powered software available by the end of 2024.
Back in November 2023, Elucid raised $80 million in Series C funding to ramp up commercialization for PlaqueIQ.