FDA clears credit card-sized heart monitor
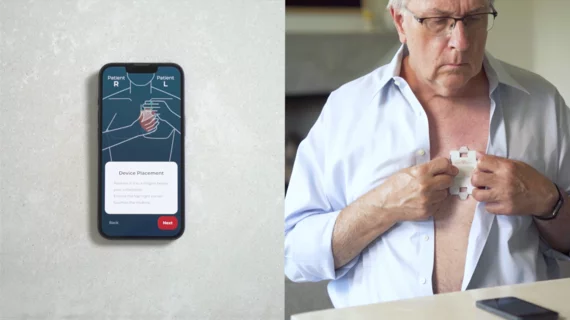
HeartBeam, a California-based healthcare technology company, has received U.S. Food and Drug Administration (FDA) clearance for its cable-free, credit card-sized heart monitor that produces 12-lead electrocardiograms (ECGs).
The new device was designed to help patients evaluate their own heart health in real time. When symptoms begin, they place the device using guidance from the HeartBeam app and then capture 30 seconds of data. That data is then automatically sent to the cloud and processed for a trained physician to review.
“It’s well documented that patients who delay seeking care for their cardiac symptoms face worse clinical outcomes,” HeartBeam CEO Robert Eno said in a statement. “The ability for patients to capture high-fidelity ECG signals from three directions wherever they are when symptoms occur will help patients get the care they need in a timelier manner. The FDA clearance of our technology is a significant milestone for the company that brings us one step closer to fulfilling our vision of providing unprecedented cardiac insights to individuals and physicians.”
According to HeartBeam, this FDA clearance represents the foundation of any future submissions it may in the future. It will continue to pursue approvals focused on synthesizing high-quality ECGs, artificial intelligence (AI)-enabled disease detection and identifying patients who face an increased myocardial infarction risk.
The company is especially encouraged by its potential to turn high-quality ECG data into AI-powered risk assessments, describing the technology as a “transformative opportunity” to improve care for heart patients all over the world.
Research underway on technology’s potential
In May, HeartBeam announced the launch of its VALID-ECG study, which will track the use of its heart monitor technology in nearly 200 patients.
“The initiation of the VALID-ECG study is a major milestone for the company and a reflection of our commitment to provide a strong foundation of clinical data as we strive to provide patients and physicians with the ability to accurately monitor cardiac disease outside of a medical facility,” Branislav Vajdic, PhD, president and founder of HeartBeam, said at the time. “In addition, our product pipeline includes coupling AI with our data-rich 3D VECG technology which will enable us to extract unique information and longitudinal insights to transform how cardiac care is monitored in the future.”