Policy & Regulations
This channel includes news coverage of healthcare policy and regulations set by Congress, the states, Centers for Disease Control and Prevention (CDC), the Department of Health and Human Services (HHS), U.S. Food and Drug Administration (FDA), and medical associations and societies.
Displaying 1 - 8 of 473
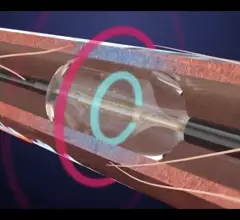
![The use of intravascular lithotripsy (IVL) during percutaneous coronary intervention (PCI) is still safe and effective when patients present with calcified nodules (CNs), according to new long-term data published in EuroIntervention.[1] Researchers compared outcomes from patients with and without CNs, highlighting key similarities in stent expansion and luminal gain.](/sites/default/files/styles/top_stories/public/2024-12/screenshot_2024-12-02_at_11.07.21_am.png.webp?itok=YDi6SyF_)



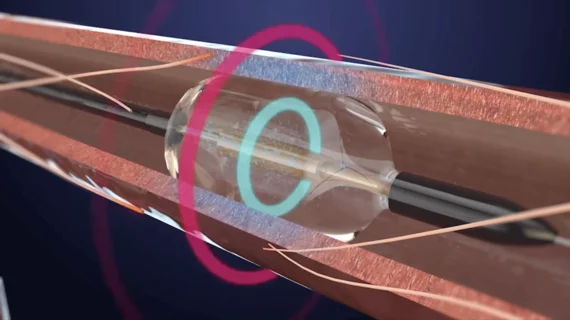
![The use of intravascular lithotripsy (IVL) during percutaneous coronary intervention (PCI) is still safe and effective when patients present with calcified nodules (CNs), according to new long-term data published in EuroIntervention.[1] Researchers compared outcomes from patients with and without CNs, highlighting key similarities in stent expansion and luminal gain.](/sites/default/files/styles/240x220/public/2024-12/screenshot_2024-12-02_at_11.07.21_am.png.webp?itok=d6Myns-z)