Peripheral Artery Disease
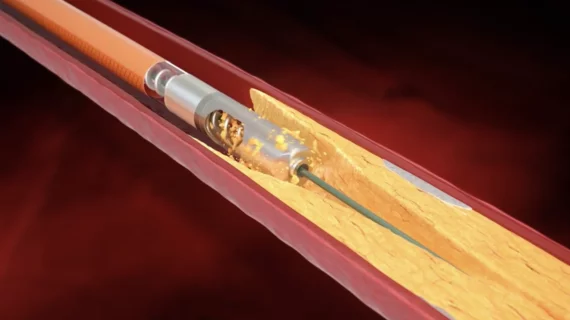
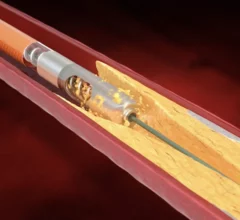
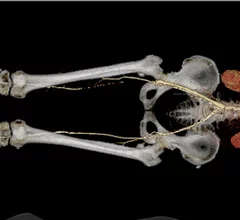
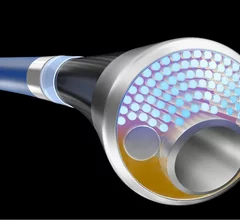
Peripheral artery disease (PAD) involves atherosclerosis mainly in the extremities, especially in the legs and feet that lead to ischemia. Untreated, PAD can progress to critical limb ischemia (CLI), also called chronic limb-threatening ischemia (CLTI), which will lead to foot or leg amputation. The mortality rate for these CLI amputees is 70% within three years. There is currently an epidemic of PAD and CLI in the U.S. The majority of patients are defined by health disparities concentrated in the Black, Latino, Native American populations in both rural and low-income urban areas. A large number of PAD patients have other comorbities, with diabetes being one a primary issue.