FDA clears AI tool that flags signs of heart disease in chest CT scans
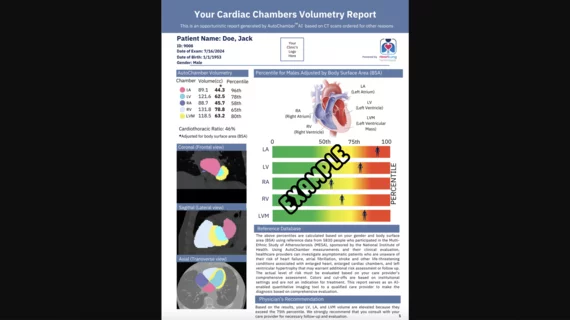
HeartLung Technologies, a Houston-based artificial intelligence (AI) company, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for new software that assesses chest CT scans for signs of coronary artery disease (CAD) and other potentially fatal heart conditions. The newly cleared offering, AutoChamber, was designed with opportunistic heart evaluations in mind.
AutoChamber can evaluate a variety of different CT images for signs of CAD, including those originally gathered to capture coronary artery calcium (CAC) scores or screen patients for lung cancer. Within 15 to 20 seconds, it builds a report that estimates and reports the volume of the patient’s cardiac chambers and flags any unusual findings for clinicians to review.
AutoChamber also works with coronary CT angiography (CCTA) scans, which have gained significant popularity among care teams in recent years.
“I feel strongly about the opportunity AutoChamber may have to evaluate cardiovascular risk for the millions of persons getting chest CT scans worldwide, in particular for lung cancer screening and other non-cardiac reasons” Nathan Wong, PhD, MPH, a professor of medicine and epidemiology at University of California, Irvine with experience using the technology, said in a statement. “The potential is for identifying those at increased risk who could benefit from earlier intervention to improve outcomes.”
“By adding AutoChamber AI to CT scans, including CAC and CCTA scans, doctors not only can evaluate coronary disease but also the risk of heart failure, atrial fibrillation and stroke, which are not reported today,” added Morteza Naghavi, MD, founder and president of HeartLung Technologies. “The opportunity is even greater in millions of lung CT scans.”
AutoChamber previously received the FDA’s breakthrough device designation, suggesting the agency saw significant potential in the technology from the start. The breakthrough devices program is designed to help medical devices make it through the approval process faster than they would otherwise. FDA representatives work directly with the device manufacturer, for example, and any submissions related to the device will be prioritized.