FDA clears new AI to guide point-of-care cardiac ultrasound exams
The U.S. Food and Drug Administration (FDA) has granted market clearance to UltraSight for its artificial intelligence (AI) guidance technology, which shows ultrasound users how to perform a diagnostic quality echocardiography exam. The UltraSight real-time AI guidance software can assist medical professionals without sonography experience in acquiring cardiac ultrasound images at the point of care in multiple settings.
This is the second AI to gain FDA approval for guiding novice users to perform cardiac ultrasound exams. The technology was created to help overcome bottlenecks within the U.S. healthcare system to get imaging, and to address the lack of training among non-sonographers and new sonographers to obtain diagnostic quality exams.
"Time to clinical decisions and clinical confidence are key aspects in patient care. This is true for people walking into the emergency department with acute heart conditions, or patients requiring ongoing monitoring," said Roberto Lang, MD, director of cardiovascular imaging at the University of Chicago. "Ultrasound is a predominant tool in patient diagnosis, but its user-dependent functionality and subjectivity in image quality assessment have been hurdles in providing care for heart patients in multiple care settings. UltraSight's real-time AI guidance is a gamechanger for diagnostic efficiency and experience. Now, with regulatory clearance, medical professionals and patients alike can benefit from this transformative cardiac imaging solution."
Lang said the technology allows anyone to perform an echo exam and produce high-quality diagnostic images. This is why the technology is initially being aimed at the point-of-care ultrasound (POCUS) market, so non-sonographers can capture the proper views needed for a cardiologist or the AI to make measurements. "It is easy to do. Actually, my grandson can now obtain an echo," Lang said.
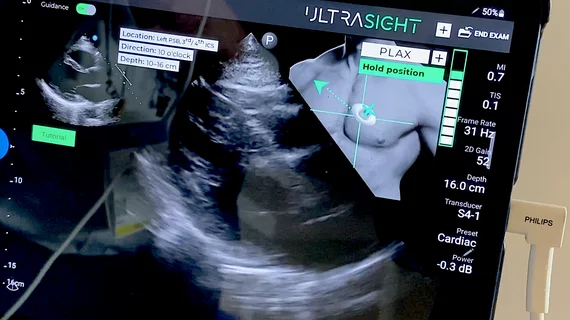
The system shows a thumbnail image in the corner of the ultrasound screen for how to position the transducer and arrows for the direction to move it. A bar graph shows the user when they are getting close or in the optimal imaging window. The AI then prompts the user to hold position, or how to move or sweep the transducer to complete the exam.
"It is something like a pilot when you see a cross where they want to throw a bomb, these systems actually tell you how to move the transducer over these crosses. It is then interesting that the system will do an auto acquisition. And then the system will use AI to make the measurements on that acquisition. It is an unbelievable thing when you see it working," Lang explained.
UltraSight said the solution will enable hospital staff to advance patient triage and treatment with increased efficiency and clinical confidence. It can also increase access to care for chronic heart disease patients by bringing cardiac ultrasound into local communities, potentially increasing patient adherence to critical treatments.
"The issues arising from the disproportion between the number of heart disease patients and availability of cardiac ultrasound was a key driver for the company's founding team," Davidi Vortman, CEO of UltraSight, said in a statement from the company. "The need to solve this significant disparity is why we applied deep geometrical machine-learning techniques to cardiac ultrasound, and what we found is that AI has the potential to close the skillset gap—empowering medical professionals to successfully acquire timely and accurate cardiac ultrasound images anywhere. With FDA clearance, we can now move forward with bringing our innovation to market and ultimately advancing patient care for the millions in need."
UltraSight's AI Guidance software is indicated for use in two-dimensional transthoracic echocardiography (2D-TTE) for adult patients, specifically in the acquisition of the 10 standard views of the heart. The company's submission for FDA clearance was based on its landmark pivotal study, which demonstrated that with real-time guidance of the ultrasound probe and feedback on the quality of the ultrasound image, medical professionals without prior ultrasound experience can acquire diagnostic quality images.
UltraSight's software is designed as an accessory for POCUS systems and is compatible with the Philips Lumify Ultrasound System. When paired with a compatible device, UltraSight's underlying AI neural network predicts the position of the ultrasound probe relative to the heart, based on the ultrasound video stream, and guides the user on maneuvering the probe to capture diagnostic quality cardiac images.
The company has a European CE mark for its real-time guidance software for cardiac ultrasound in the EU and UKCA marking in the U.K. In 2022, UltraSight won the Bristol Myers Squibb Improving Cardiovascular Disease Outcomes Challenge as the most "innovative cardiac technology."
UltraSight was a partner vendor with the American Society of Echocardiography (ASE) for its annual meeting in June.