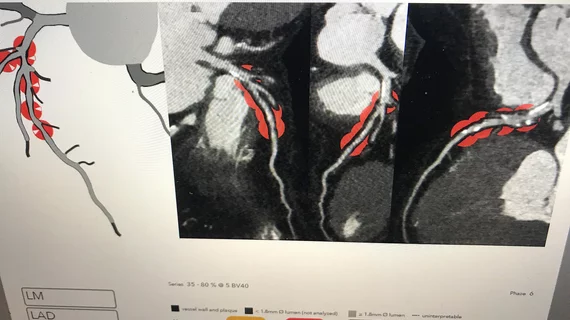
HeartFlow announces US launch of AI-powered solution for evaluating coronary arteries
HeartFlow has announced the U.S. launch of RoadMap Analysis, a new artificial intelligence (AI) solution able to identify stenoses in patients with suspected coronary artery disease (CAD) who undergo cardiac CT imaging. The tool’s AI capabilities were trained to evaluate the location and severity of any potential issues, which can save clinicians time and help them deliver accurate diagnoses.
The California-based healthcare technology company shared the news April 17.
“As a leader in non-invasive diagnosis of CAD, HeartFlow is intimately familiar with the challenges associated with diagnosing heart disease,” HeartFlow CEO John Farquhar said in a prepared statement. “The RoadMap Analysis underscores our commitment to delivering products that meet the needs of physicians and ultimately helps deliver improved treatment plans for patients with suspected CAD—no matter how their disease may present itself.”
“The RoadMap Analysis has already been a valuable tool for our organization in helping us better pinpoint areas of stenosis and its severity,” Michael Morris, MD, a radiologist with Banner Health, added in the same statement. “It helps our providers confidently determine the best next steps necessary to achieve improved patient care and outcomes.”
HeartFlow first received U.S. Food and Drug Administration (FDA) clearance for RoadMap analysis back in October 2022. The company also gained FDA clearance for another AI-powered solution, Plaque Analysis, at the same time.
The news comes just days after HeartFlow announced the completion of a Series F funding round worth $215 million by its parent company, HeartFlow Holding. The funding round was led by Bain Capital Life Sciences.
“We look forward to supporting the company’s commitment to improving cardiovascular care for patients as it heads into this exciting next chapter of growth,” Nicholas Downing, MD, a managing director at Bain Capital Life Sciences, said at the time.
Click here for the full story.