Succeeding with Cancer: Using Imaging to Avoid Treatment-induced Heart Failure
Treating today’s cancer patient no longer means simply targeting the cancer. Given the known cardiotoxicities of some established chemotherapies and the possibility that newer approaches may damage the heart, oncologists, cardiologists and imaging specialists now work together to detect and minimize the risk of treatment-induced heart failure.
Echocardiography can be used to help monitor cardiac toxicity using a baseline cardiac ultrasound and serial followup exams.
Anthracycline chemo agents can cause damage to the heart
Anthracyclines have long been oncologists’ first line of attack against a broad array of solid tumors and hematological malignancies because of their effectiveness. While anthracycline chemo agents destroy cancer cells, they also may induce cardiomyocyte death that leads to adverse left ventricular remodeling. Chemotherapy agents can cause cardiomyopathy and heart failure.
To prevent the risk of patients developing heart failure, oncologists now typically monitor left ventricular ejection fraction (LVEF) using either echocardiography or equilibrium radionuclide angiography/multigated acquisition (ERNA/MUGA) and discontinue or pause treatment if LVEF measures drop below a threshold.
Anthracyclines, probably the most commonly prescribed type of anticancer agent, are being joined by an expanding array of therapies, says Raymond R. Russell, MD, PhD, director of nuclear cardiology at the Cardiovascular Institute at Rhode Island Hospital in Providence and director of its cardio-oncology program. His research has helped elucidate the cellular pathways for anthracycline-induced heart failure.
Besides anthracyclines, oncologists may consider conventional therapies such as taxanes, an antimicrotubule agent, and radiation. Newer approaches include biological drugs such as trastuzumab, a monoclonal antibody; 5-fluorouracil, an antimetabolite; small molecule tyrosine kinase inhibitors; proteasome inhibitors; and immune therapy. Each is designed to exploit a potential vulnerability in cancer cells, widening physicians’ arsenal against the disease. And each carries its own potential, and sometimes as yet unknown drawbacks.
“There have been dramatic changes in what is being done for cancer, [but] there are some unique forms of cardiotoxicity with different agents,” says Russell, the lead author of an information statement from the American Society of Nuclear Cardiology (ASNC) on multimodality imaging and its role in the management of cardiac complications in patients under treatment for cancer (J Nucl Cardiol 2016;23[4]:856-84).
Cardiovascular risk factors and individual variability add other layers of complexity. Cumulative dose is the largest predictor of cardiotoxicity with anthracyclines, for instance, but some patients may develop LV dysfunction after one treatment and others may fare relatively well through a full cycle. The challenge with anthracyclines and other options is identifying who is at risk, or if complications develop, intervening early enough to reverse or minimize the development of heart failure.
“Part of [the risk of developing heart failure] is due to the cancer treatment, but there definitely is individual susceptibility,” says Ana Barac, MD, PhD, a cardiologist at MedStar Washington Hospital Center in Washington, D.C., who specializes in cardiac imaging. “There is also an increasing role of cardiovascular risk factors, in particular hypertension. While there are cancer treatments that are known to cause cardiomyopathy and lead to heart failure, there is this larger unknown yet to be discovered of individual susceptibility.”
Imaging’s role in assessing myocardial damage from chemotherapy agents
Cardio-oncology programs like the one Russell helped build at Smilow Cancer Hospital at Yale New Haven in Connecticut and later at the Cardiovascular Institute at Rhode Island emphasize a collaborative and integrated approach to care that includes imaging to promote cancer patients’ cardiovascular health. “Each test has its strengths and weaknesses,” Russell says. Cardio-oncology programs “understand the broad arsenal of imaging.”
The ASNC statement recommends multimodality imaging with nuclear cardiology techniques recognized as the gold standard of care for reducing the incidence and severity of heart failure. For instance, guidelines support the use of ERNA for monitoring patients on anthracyclines based on proven clinical effectiveness, but acknowledge radionuclide angiography exposes patients to diagnostic radiation. The American Society of Clinical Oncology’s guidelines, on which Barac is a co-author, recommend echocardiography during and after cancer treatment but, if echo is not available or feasible, then cardiac MRI or MUGA (J Clin Oncol online Dec. 5, 2016). But echo has its limitations, too, including technique-related variability.
It’s important to stick with one modality, the experts say, as long as it is reliable, reproducible and accurately measures changes over time. ASNC recognizes that numerous protocols exist with varying frequency for testing but, at least for anthracyclines and trastuzumab, all call for a baseline LVEF and serial monitoring. Some new, targeted therapies may not even require a baseline LVEF assessment, Barac says, and tipping points for intervening in time to avoid irreversible damage have yet to be established.
“We know that there are cardiac structure and function parameters that can be measured by echo or MRI or MUGA, and we know they all may start changing,” she says. “When is that key point of the change that will lead to clinical events? We don’t have a good answer.”
Russell also sees many fundamental questions outstanding. “Can we identify individuals who are at higher risk of cardiotoxicity?” In addition to noninvasive imaging, the answer may involve incorporating other tools such as biomarkers and genetic factors, he says. “With that information, what can we do to enhance cardioprotection? … The studies are a little messy here.”
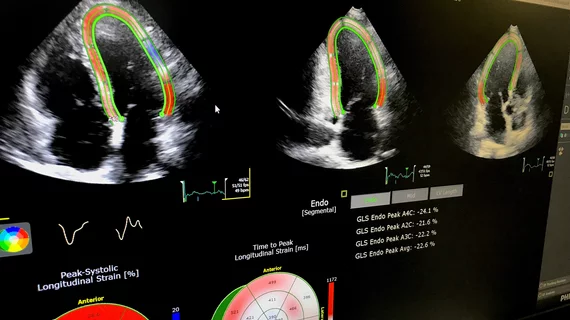
Assessing cardio-toxicity from chemotherapy agents using cardiac ultrasound
Reduced LVEF serves as a flag to oncologists and cardiologists that a cancer patient’s heart has been caught in the crossfire between a cancer treatment and its target. But LVEF also is a laggard as an indicator; at that point, the flag may be a sign of surrender, leaving physicians to try to limit the damage rather than prevent its occurrence.
“The difficulty is that the technique itself does not allow you to recognize the small changes in ejection fraction,” says Juan Carlos Plana, MD, director of the Cardio-Oncology Center at Baylor St. Luke’s Medical Center in Houston, co-director of its Center for Advanced Cardiac Imaging and a co-author with Barac on an American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging consensus statement on the use of multimodality imaging and cancer (J Am Soc Echocardiogr 2014;27[9]:911-39).
His research focuses on the early detection of cancer treatment-induced cardiac dysfunction using echo techniques, and particularly deformation imaging such as echo-derived myocardial strain. Stain can be used to detect subclinical ventricular dysfunction in patients undergoing cancer treatments and potentially may be a diagnostic and prognostic tool. The goal is to use these tools to identify patients who would benefit from cardioprotective medications such as beta-blockers or ACE inhibitors.
“The changes in strain and ejection fraction go hand in hand; they go in parallel,” Plana says. “What happens with strain is that it is like holding a magnifying glass on the changes. It is much easier to recognize.”
To date, the studies involving strain-based approaches have been small. Plana and colleagues analyzed 35 of these studies with a total of 1,504 patients and found all showed that changes in myocardial deformation preceded changes in LVEF. During treatment, an early drop in global longitudinal strain measured by speckle tracking echo of 10 percent to 15 percent predicted later cardiotoxicity (J Am Coll Cardiol 2014;63:2751–68).
Echocardiographic myocardial strain imaging for is cost effective for early detection of cardiac damage
The researchers also conducted a cost-effectiveness study on cardioprotective strategies with three scenarios in patients treated for breast cancer: an EF-guided strategy, universal cardioprotective therapy for all patients and a strain-guided strategy. Over a five-year period, the strain-based approach cost the least ($4,159 vs. $5,967 for universal care and $7,033 for EF-guided care) and beat out the others for quality-adjusted life years, too (Int J Cardiol 2016;212:336-45).
Russell and Barac agree that strain looks promising, as does the use of biomarkers. Russell also sees potential in some new radiotracers, such as ones that detect cardiac amyloidosis. But the strain-based techniques remain a work in progress. The lack of consistency among vendors is a barrier, Russell says, and Plana cautions that the technique has a learning curve.
“There are a lot of potential pitfalls so you have to have a team that is very well versed and trained in the interpretation,” Plana says. “But if you do pay attention to all these things the technique functions very well. It will allow you to recognize damage much earlier.”
Until cancer treatments can be effective yet benign for the heart, imaging will continue to play a critical role in many cancer patients’ care. Through consensus statements, multidisciplinary guidelines and collaborative cardio-oncology programs, cardiologists, oncologists and imagers at the forefront will strive to ensure cancer patients can maximize the benefits of cancer therapies with appropriate monitoring to ensure their safety, they say.
It is a challenging mission, though. “There are a lot of unknowns as to optimum care,” Russell says.
Related Cancer Therapy Cardiotoxicity Content:
Providers must rethink traditional imaging approaches to prevent cardiotoxicity in cancer patients
CV programs struggling to keep up with growing demand for cardio-oncologists
Machine learning predicts drug cardiotoxicity
Prior cardiotoxicity linked to 30% increased risk of CHF during pregnancy
CV outcomes underreported in pivotal anticancer trials
CDK2 inhibitors protect cancer patients from anthracycline-induced cardiotoxicity
Genetic variants could be key to identifying chemo-induced cardiotoxicity
T2 mapping may uncover cardiotoxic marker early enough to prevent heart failure
Some chemo drugs might be more heart-safe than others
Cardiac MRI-derived T2 mapping may help heart failure patients
Genetic variant linked to chemotherapy-induced cardiomyopathy
Study calls for better collaboration between cardiologists, oncologists
Cardiac monitoring may protect high-risk breast cancer patients against heart failure