New medical device helps cardiologists limit esophageal injuries during RF ablation procedures
A new-look medical device may reduce the risk of injuries to the esophagus during radiofrequency ablation (RFA) procedures for atrial fibrillation (AFib), according to new research published in JACC: Clinical Electrophysiology.[1]
“The incidence of esophageal ulcerations has been reported as high as 47% of patients who undergo AFib ablation,” wrote senior author Emile Daoud, MD, an electrophysiologist with Ohio State Wexner Medical Center, and colleagues. “Currently, the standard of care is to place a temperature probe in the esophagus to provide luminal esophageal temperature (LET) monitoring. Studies have reported that ablation energy delivered within the left atrium near the esophagus significantly increases the LET. However, LET monitoring has several limitations.”
Daoud et al. aimed to evaluate the potential of new piece of equipment designed to limit esophageal injuries during AFib ablation: the Esolution device developed by S4 Medical. Esolution uses “vacuum suction and mechanical deflection” to gently move the patient’s esophagus out of the way during RFA procedures, a movement designed to limit the risk of injury. S4 Medical gained de novo clearance from the U.S. Food and Drug Administration for the device in September 2023.
The EASY AF study included 120 patients who underwent RFA for AFib from September 2021 to June 2022. Patients with a history of RFA were excluded, and all patients underwent transesophageal echocardiography or endotracheal intubation within 30 days of the procedure.
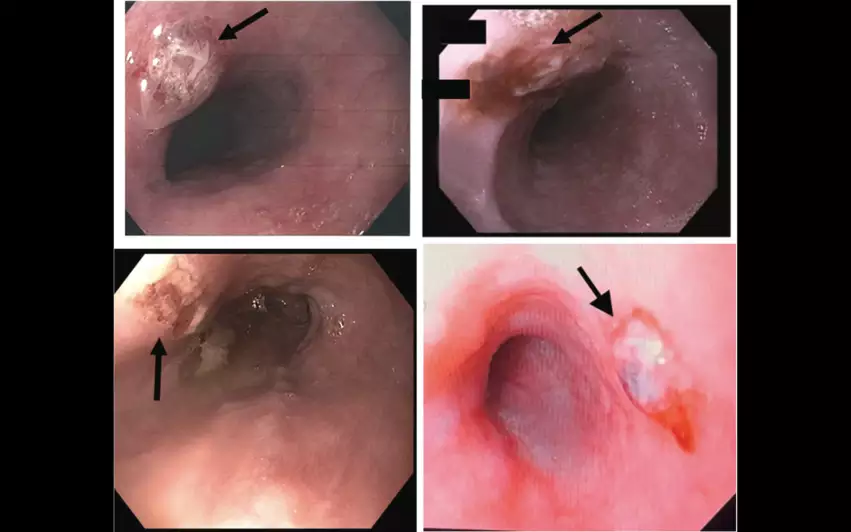
Participants were randomized to either undergo LET monitoring alone, or LET monitoring in addition to treatment with the Esolution device. While 66 patients with a mean age of 65.3 years old underwent just LET monitoring alone, serving as the study’s control group, another 54 patients with a mean age of 61.6 years old underwent LET monitoring and treatment with the new device. Within 15 to 72 hours of treatment, all patients underwent an esophageal endoscopy performed by a gastroenterologist blinded to how each patient was treated. The gastroenterologist evaluated each patient for signs of esophageal lesions.
Overall, the rate of ablation injuries to the esophageal mucosa was significantly less in the treatment group (5.7%) than the control group (35.4%). The number of ablation lesions per patient was also much higher for patients in the control group. In fact, the difference in outcomes between the two treatment strategies was so different that the EASY AF study was ended prematurely.
From a safety perspective, the authors added, the Esolution device was not associated with any adverse events.
“This study demonstrates that the deviating device, combining vacuum suction to manage the trailing edge coupled with mechanical deflection, can be easily implemented in consecutive patients with a reduction in esophageal lesions and without adverse events,” the authors wrote. “With this simple technique, routine use of a deviating device seems prudent and offers greater confidence for safe AFib ablation.”
This analysis was funded by S4 Medical, the company behind the Esolution device. Daoud helped develop the Esolution device, and he and The Ohio State University both have equity in S4 Medical. In addition, three other co-authors are members of the S4 Medical advisory board.
Click here to read the full analysis in JACC: Clinical Electrophysiology, a journal of the American College of Cardiology.