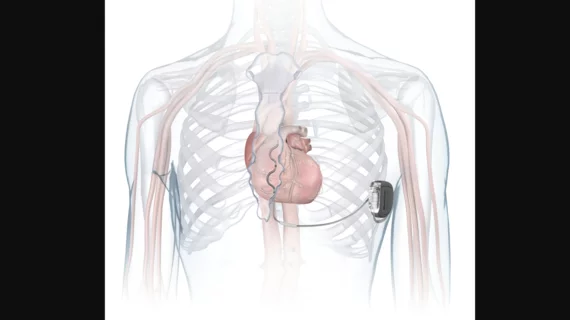
FDA approves Medtronic’s extravascular ICD for abnormal heart rhythms
Medtronic has gained U.S. Food and Drug Administration (FDA) approval for a new first-of-its-kind extravascular implantable cardioverter-defibrillator (ICD) and defibrillation lead that allows cardiologists to treat abnormal heart rhythms from outside of the patient’s heart and veins.
The Aurora EV-ICD MRI SureScan is similar in size and shape to traditional ICDs, but it’s implanted below the left armpit. The Epsila EV MRI SureScan lead is then placed under the breastbone and outside of the heart and veins in a minimally invasive procedure designed to limit the risk of vascular injuries and other issues associated with the use of traditional transvenous leads. This is the first ICD commercially available to U.S. patients that is placed outside of the vascular space. It’s capable of defibrillation, anti-tachycardia pacing and back-up pacing therapies.
The FDA largely based its decision on evidence that emerged in the Medtronic-funded Extravascular ICD Pivotal Study. Findings from that analysis were published in The New England Journal of Medicine in October 2022, and follow-up research based on those findings was presented at the Heart Rhythm Society’s Heart Rhythm 2023 conference in New Orleans.
“The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology,” Bradley P. Knight, MD, medical director of electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute and a researcher with experience evaluating the newly approved device, said in a prepared statement. “Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology.”
“This FDA approval paves the way for patients to have a better overall experience with ICD therapy,” added Alan Cheng, MD, chief medical officer of Medtronic’s cardiac rhythm management business. “ICDs remain the gold standard for prevention of sudden cardiac death, and while the subcutaneous ICD avoids certain complications associated with transvenous defibrillators, it has limitations that may affect a patient's comfort and quality-of-life.”
The Aurora EV-ICD MRI SureScan and Epsila EV MRI SureScan defibrillation leads previously gained CE mark approval back in February 2023. They are expected to be commercially available in the United States on a limited basis in the weeks ahead.