Heart rhythm specialists raise $7.2M to fund advances for FDA-cleared ECG system
CathVision, a Denmark-based healthcare technology company focused on the electrophysiology (EP) lab, has closed a new financing round worth $7.2 million. The funding all came from existing investors.
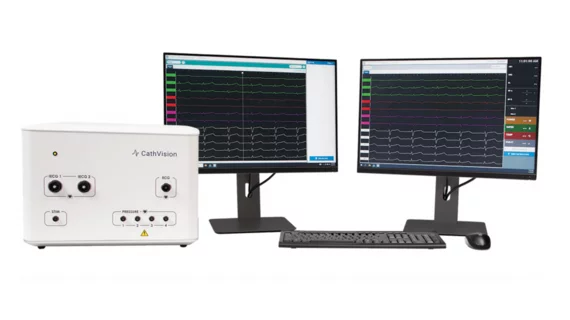
This influx of financing is expected to go toward pushing the development and commercialization of CathVision’s ECGenius EP Recording System, a new solution designed to capture high-fidelity, low-noise electrocardiogram (ECG) recordings for the diagnosis and treatment of arrhythmias such as atrial fibrillation (AFib).
The ECGenius system received FDA clearance in May 2022. It is currently available in the United States on a limited basis, but not yet available in the European Union. In the near future, CathVision anticipates adding advanced artificial intelligence (AI) capabilities to the system to improve its effectiveness.
“Our investors have witnessed the evolution of our company and technology in recent years,” Mads Matthiesen, CathVision’s CEO, said in a prepared statement. “Reinvesting in the company demonstrates confidence in our innovation, ongoing development, and ability to advance adoption of the ECGenius System throughout the United States as we focus on empowering physicians to diagnose, characterize, and treat cardiac arrhythmias more effectively. We are expanding our sales team, building our U.S. presence, and furthering the development of analytic modules.”
“Cardiac ablation is one of the fastest growing markets in healthcare; treatment efficiency and efficacy must improve for physicians to deliver the best possible care for patients with atrial fibrillation and other cardiac arrhythmias,” Matthieu Bocquet, a founding partner of Lumine Capital, said in the same statement. “CathVision’s modern and precise EP recording technology brings more clarity to physicians by delivering clear signals that can be accurately interpreted. This is a necessary step to improve treatment outcomes.”