FDA grants drug-eluting bioadaptor its breakthrough device designation
Elixir Medical, a California-based healthcare technology company focused on coronary and peripheral artery disease, received the FDA’s breakthrough device designation for its DynamX sirolimus-eluting coronary bioadaptor. The designation is for improving coronary luminal diameter, restoring hemodynamic modulation and reducing plaque progression in patients with symptomatic ischemic heart disease related to de novo coronary artery lesions.
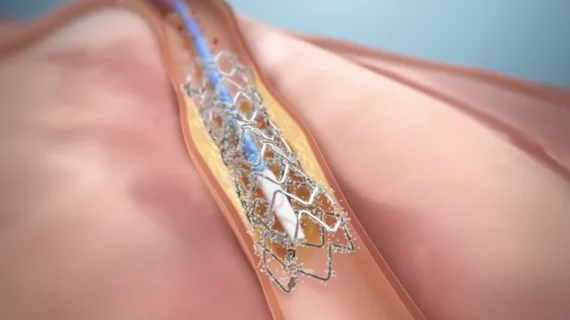
The DynamX system was designed to help return diseased vessels “to a more normal condition” through a series of phases. First, Elixir Medical explained, the device determines a patient’s maximum flow lumen and restores blood flow. This is followed by a second phase during which the bioadaptor “is encapsulated with tissue and the absorbable polymer coating is resorbed.” Finally, once the vessel is released, it receives “adaptive dynamic support” that designed to restore viability.
According to two-year data presented at EuroPCR 2024 in Paris, use of the DynamX bioadaptor is associated with a 65% reduction in target lesion failure and fewer adverse events compared to normal care.
This device is still not approved by the FDA to be sold and marketed in the United States The FDA’s breakthrough devices program is designed to help medical devices make it through the approval process faster than they would otherwise. The agency’s representatives work directly with the device manufacturer, for example, and any submissions related to the device will be prioritized.
“For many years, it was thought that caging of the vessel with stents was the main driver for annual increasing non-plateauing event rates,” Motasim Sirhan, CEO of Elixir Medical, said in a statement. “However, the data with the ‘leave nothing behind’ approach with bioresorbable scaffold technologies showed non-plateauing event rates continue to occur even after resorption of the scaffold, so we needed a more innovative approach to restore vessel viability. We very much appreciate FDA’s breakthrough designation recognition of the bioadaptor technology.”
So far 2024 has been a busy year for Elixir Medical. In March, its DynamX BTK System, an implantable device for treating below-the-knee blood vessels in chronic limb-threatening ischemia patients, received the FDA’s breakthrough device designation.