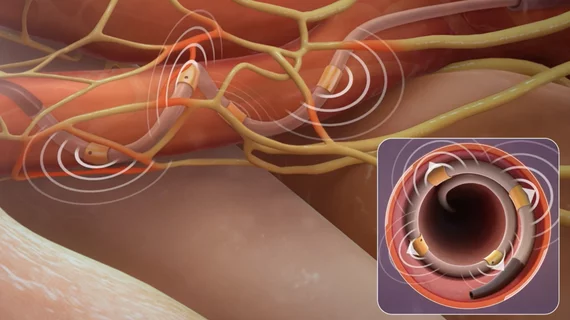
First US patient treated with Medtronic’s FDA-approved renal denervation system
The first U.S. patient has undergone treatment with Medtronic’s Symplicity Spyral renal denervation (RDN) system for hypertension. The news comes just days after the U.S. Food and Drug Administration (FDA) approved the device.
David Kandzari, MD, an interventional cardiologist with Piedmont Atlanta Hospital, who served as principal investigator of the SPYRAL HTN-ON MED clinical trial, performed the RDN procedure. His experience with Symplicity Spyral made him the best possible person to debut the device in a clinical setting, according to Medtronic.
“It’s an exciting opportunity to begin this new chapter of hypertension treatment with the first U.S. procedure using the Symplicity Spyral RDN system,” Kandzari said in a statement. “For people with hypertension, medication and/or lifestyle changes can help reduce blood pressure, but studies have shown that many people still don’t gain control over their condition. I am inspired by the potential of this complementary therapy to help treat the issue of high blood pressure for so many patients.”
“This milestone brings potential hope to millions of Americans who might want another treatment option—other than medication and lifestyle changes—to better manage their blood pressure,” Jason Weidman, senior vice president and president of the Coronary and Renal Denervation business with Medtronic, added in the same statement. “We want to acknowledge everyone who has continued to back this therapy along the journey, and we’re excited for the future of hypertension care.”
A dramatic few months for Medtronic’s Symplicity Spyral RDN system
When the FDA announced its approval of the Symplicity Spyral system, it came as a bit of a surprise to some in the industry after an FDA advisory panel had voted not to endorse the device in August.
The panel voted 13 to 0 that the device was safe to use for patients with uncontrolled hypertension, and it voted 7 to 6 that the device was effective for treating the intended patient population. However, the group could not agree that the RDN system’s benefits outweigh any potential risks.
“I think we have to be cautious not to confound the desperateness of the unmet need with a willingness to throw anything at that unmet need,” panelist Julia B. Lewis, MD, a professor of medicine with Vanderbilt Health, said at the time. “It’s not going to help people’s blood pressure and their cardiovascular outcomes to have a procedure that is potentially either ineffective or minimally effective.”
Of course, the FDA’s final decisions do not always line up with the opinions of its advisory panels. On Nov. 17, Medtronic announced that Symplicity Spyral was officially the second RDN system for hypertension to gain full approval in the United States.
"Medtronic has always believed in the potential of this therapy,” Weidman said at the time.