Medtronic expands carotid portfolio with Contego Medical deal
Medtronic has entered into an exclusive U.S. distribution agreement with Contego Medical to distribute its products for carotid and peripheral vascular disease revascularization.
Medtronic has held a minority investment in the North Carolina-based company since 2020. This agreement includes an increased investment and gives Medtronic the option to acquire Contego Medical in full at a later date.
This move is part of a larger trend in interventional cardiology where there is growing procedure volume in carotid stenting. This stems from an October 2023 change in the Medicare carotid artery stenting national coverage determination (NCD) that increased the number of patients eligible for reimbursement. Experts predicted this would results in rising CAS procedure volumes.
“Contego’s innovations, backed by excellent data, are transforming how carotid disease is treated and complement the Medtronic peripheral and stroke protection portfolio," David Moeller, senior vice president and president of peripheral vascular health for Medtronic, said in a statement. "This strategic agreement with Contego Medical expands our commitment to this fast-growing carotid market.”
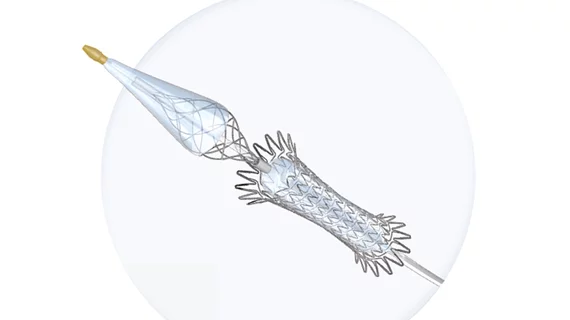
This agreement includes Contego Medical’s portfolio of commercially available products, including the Excipio peripheral thrombectomy devices and the recently FDA-cleared Neuroguard carotid stenting system.
The Neuroguard IEP system combines a stent, post-dilation balloon and an integrated embolic protection filter. According to Contego Medical, the PERFORMANCE I trial and PERFORMANCE II IDE trial consistently recorded unprecedented low event rates, with no major strokes, zero neurologic deaths and no stent thromboses for up to two-year after treatment with the Neuroguard IEP system. The company is currently evaluating the Neuroguard IEP system with a next-generation direct transcarotid access and protection system, TCAR-IEP, for advanced stroke protection regardless of vascular approach in the PERFORMANCE III trial, but it is not commercially available in the U.S.
“This strategic agreement marks a significant milestone for both Contego Medical and Medtronic. By combining our innovative product portfolio with Medtronic’s extensive market presence and clinical leadership, we are positioned to revolutionize revascularization treatment in the carotid and peripheral vascular disease space," Ravish Sachar, MD, founder and CEO of Contego Medical, said in the same statement. “This partnership enhances our ability to deliver state-of-the-art solutions to patients and reinforces our commitment to expanding access across the United States.”
Medtronic said it plans to start distributing Contego Medical's portfolio of products by spring 2025.