Interventional Cardiology
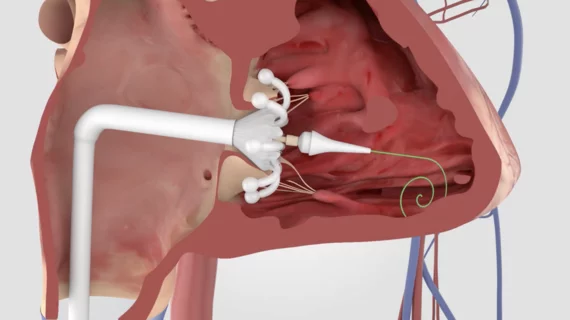
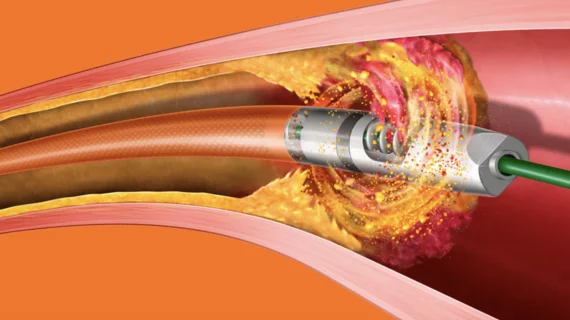
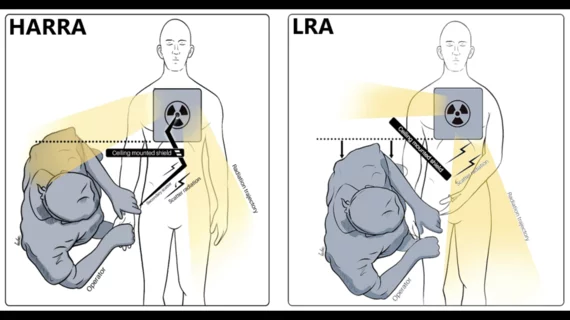
This cardiac subspecialty uses minimally invasive, catheter-based technologies in a cath lab to diagnose and treat coronary artery disease (CAD). The main focus in on percutaneous coronary interventions (PCI) to revascularize patients with CAD that is causing blockages resulting in ischemia or myocardial infarction. PCI mainly consists of angioplasty and implanting stents. Interventional cardiology has greatly expanded in scope over recent years to include a number of transcatheter structural heart interventions.
Displaying 9 - 16 of 2775