Left Atrial Appendage Closure
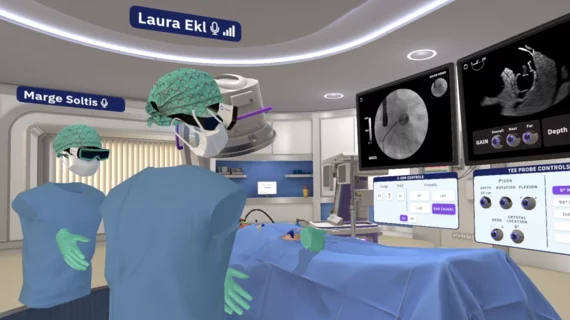
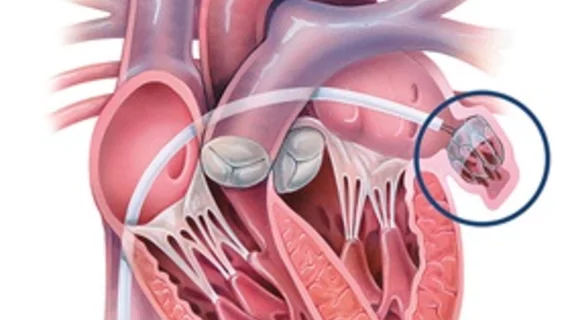
The left atrial appendage (LAA) of the heart is a common location where clots form in atrial fibrillation (AFib) patients that then embolism and cause a stroke. Closing off the LAA either surgically, with a clip or via transcatheter LAA closure device, closes the opening to the LAA to prevent clots from forming or embolizing. The clots are the reason why AFib patients need to be on anticoagulants. LAA occlusion (LAAO) enables patients to stop taking anticoagulation drugs. LAAO has been a rapidly growing segment of structural heart procedures since the approval of the first device, the Watchman, in 2015. Procedures are performed by electrophysiologists, interventional cardiologist or cardiac surgeons.
![Left atrial appendage occlusion (LAAO) with the Watchman FLX device from Boston Scientific is associated with positive outcomes and limited adverse events after one year, according to new findings published in Circulation: Cardiovascular Interventions.[1] Many prior Watchman FLX studies, including PINNACLE FLX, had focused on the device’s performance in a controlled setting. The study’s authors hoped to gain a better understanding of its real-world impact by reviewing registry data from more than 97,000 U.S](/sites/default/files/styles/top_stories/public/2024-08/screenshot_2024-08-12_at_11.35.13_am.png.webp?itok=5FeQeMFj)