Medical device company raises $104M to fund PFA research
Kardium, a Canadian medical device company focused on electrophysiology technologies, has raised $104 million in new financing. Fidelity Management and Research Company led the funding round; T. Rowe Price Associates and new investor Durable Capital Partners also participated.
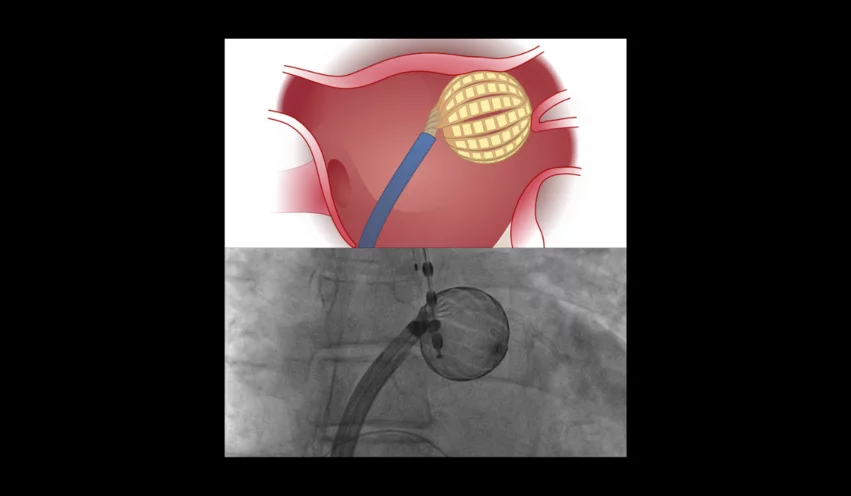
The new financing is expected to go toward funding additional research into the safety and effectiveness of Kardium’s Globe Pulsed Field Mapping and Ablation System for patients with atrial fibrillation (AFib).
“We are very excited to have the continued support of existing investors, and welcome Durable Capital Partners to this round,” Kevin Chaplin, CEO of Kardium, said in a statement. “Their enthusiasm and support reflect the incredible success we have achieved with the Globe System and the tremendous potential we have to dramatically improve the treatment of AFib for millions of patients worldwide.”
Financing push follows positive data
Back in May, early data on the Globe system was presented at Heart Rhythm 2024 in Boston and simultaneously published in Heart Rhythm. The first-in-human study found that the new-look pulsed field ablation (PFA) device was associated with a 100% success rate for pulmonary vein isolation, 100% success rate for the posterior walls and 91% success rate for the mitral isthmuses. Mean pulse delivery time, left atrial catheter dwell time, fluoroscopy time and procedure time were 61.5 seconds, 53.9 minutes, 5.3 minutes and 87.8 minutes, respectively. Freedom from atrial arrhythmia after one year was 84.2% for patients with paroxysmal AFib and 80% for those with persistent AFib.
“I am very excited by the results of the PULSE-EU Study with the Globe Pulsed Field System,” Vivek Reddy, MD, director of cardiac arrhythmia service with The Mount Sinai Hospital in New York City, said at the time. “The Globe System achieved outstanding freedom from atrial arrhythmia in both paroxysmal and persistent patients, with no device- or procedure-related major adverse events. These outcomes underscore the tremendous potential of the Globe System in advancing AFib treatment and improving patient outcomes.”