FDA announces yet another recall, the fifth of 2023, for troubled heart devices
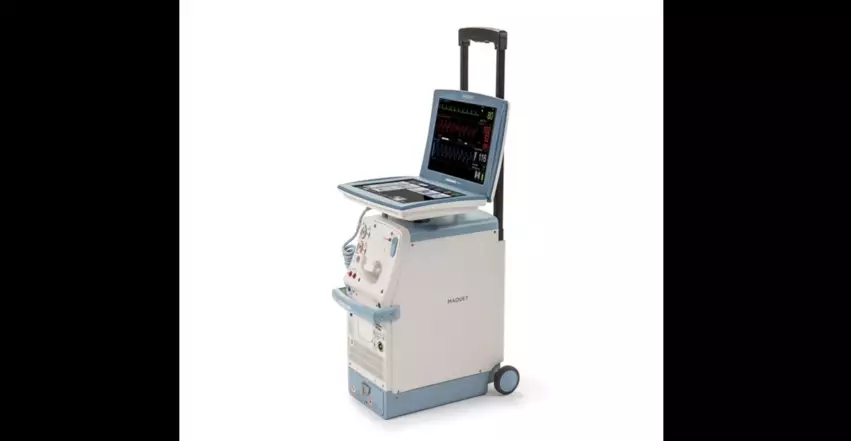
The U.S. Food and Drug Administration (FDA) has announced that Datascope, a subsidiary of Getinge, is once again recalling certain Cardiosave intra-aortic balloon pumps (IABPs) due to a risk that the devices could stop working because of mechanical failures. This is a Class I recall, with use of these IABPs potentially resulting in “serious injuries or death.”
These devices are designed to provide temporary support to a patient’s left ventricle. Due to issues with the printed circuit board assembly, however, the batteries may fail to properly charge. According to the FDA’s advisory, using an affected device “may cause serious adverse health events, including unstable blood pressure, injury (for example: inadequate blood supply or a vital organ injury), and death.”
The recall includes 4,586 Cardiosave Hybrid IABPs and Cardiosave Rescue IABPs distributed to U.S. customers from March 2012 to May 2023. There have been 252 complaints about the issue, but no injuries or deaths have been reported.
Datascope sent customers impacted by this recall an Important Medical Device Advisory Letter, which recommends certain steps that prevent electrical surges when charging the device’s batteries. Batteries should not be removed when the power level is at 80% or higher and actively charging, for example. If it is “absolutely necessary” to do so, the Datascope document recommends connecting the catheter to an alternative IABP, turning the IABP off by pressing and holding the power button and unplugging the device. Then, according to the document, it may be safe to remove the battery if following all other instructions.
A history of significant issues with the Cardiosave Hybrid and Rescue IABPs
This announcement represents the fifth recall in 2023 alone for the Cardiosave Hybrid and Cardiosave Rescue IABPs. The first, announced in January, was due to a risk of blood entering the IABP through a damaged catheter. The second, third and fourth recalls of 2023 were all related to a heightened risk that the devices may stop working unexpectedly, just like this most recent incident.
In December 2021, meanwhile, fluid leaks led to a separate Class I recall for these devices.