FDA’s tirzepatide decision creates uncertainty for patients—and leads to a lawsuit
The Outsourcing Facilities Association (OFA), a Texas-based trade association focused on distributing compounded medications throughout the healthcare industry, has sued the U.S. Food and Drug Administration (FDA) for its decision to remove tirzepatide from the federal drug shortage list.
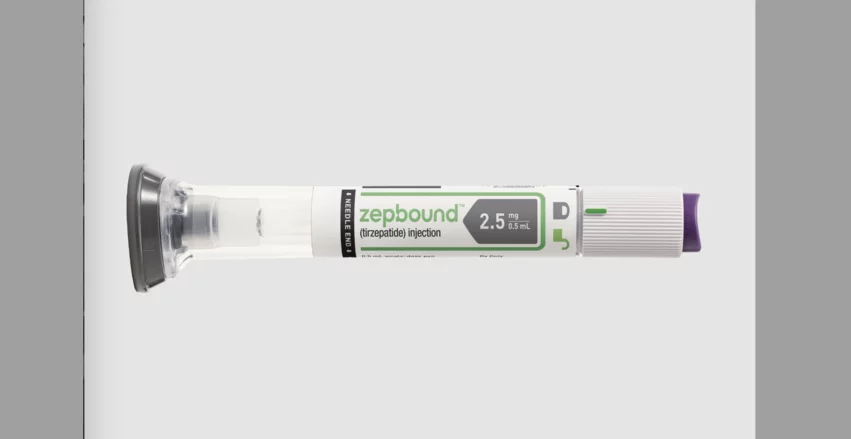
Tirzepatide is diabetes drug sold by Eli Lilly and Company under the brand names Zepbound and Mounjaro. It has also been linked to improved outcomes in patients with sleep apnea and heart failure with preserved ejection fraction.
When drugs are on the FDA’s drug shortage list, outsourcing facilities such as the ones represented by OFA have a green light to help produce their own “essential copy” versions of those drugs. Compounded drugs are not technically approved by the FDA, though they are still required to meet certain conditions predetermined by the FDA.
According to OFA’s complaint, the FDA removed tirzepatide from the drug shortage list without giving proper notice.
“The FDA’s removal of tirzepatide from the shortage list without the due process of proper federal notice, at a time when they acknowledge that shortages still exist, is the definition of arbitrary and capricious,” Lee H. Rosebush, chairman of OFA, said in a statement. “The agency’s decision will have tremendous implications across the nation for patients and physicians, as well as the outsourcing facilities that made an enormous investment to meet patient demand in light of product shortages and delays.”
These medications had been part of the shortage list for so long—since 2022, in fact—that drug manufacturers, pharmacies, physicians and patients have all grown accustomed to securing compounded version as needed. Suddenly removing these medications from the shortage list puts an end to “business as usual” for a lot of parties. CNN even explored this side effect of the FDA’s update, interviewing one patient who had found considerable success taking a compounded version of tirzepatide and may not be able to afford treatment now that she is required to take Zepbound.
Click here to read the OFA lawsuit.
Click here for information on FDA policies related to the federal drug shortage list and compounders.