Safety update: Sensor issue with Abbott CGMs ruled a Class I recall
Safety concerns associated with Abbott’s FreeStyle Libre 3 continuous glucose monitoring (CGM) system have been categorized as a Class I recall, the U.S. Food and Drug Administration (FDA) announced to the public Thursday, Sept. 5.
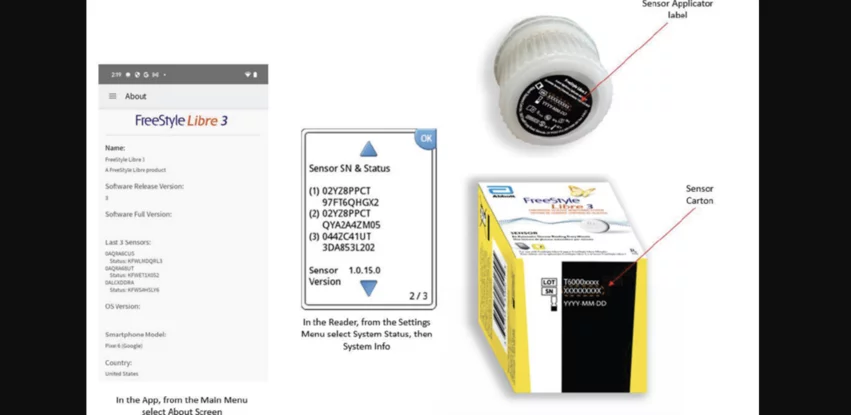
The issue, first shared by Abbott in July 2024, involves the sensors of certain FreeStyle Libre 3 devices producing inaccurate glucose readings. Abbott issued a medical device correction at the time, warning customers and patients that the impacted sensors—from lots T60001948, T60001966 and T60001969—are not to be used.
Now, the FDA has confirmed that these concerns are a Class I recall, which means it believes the devices “may cause serious injury or death” if used for patient care.
“The use of affected product may cause serious adverse health consequences, including severe low blood sugar (hypoglycemia), which can cause central nervous system problems, loss of consciousness, seizures, coma, permanent brain damage and death,” the agency wrote.
Two injuries have been reported related to these issues. There have been no patient deaths.
The FDA’s update emphasized that all users should see if their devices are included in this recall. Detailed instructions on how to know for sure were provided. Any affected sensors can be thrown away immediately, and Abbott will send out a replacement at no added cost.
According to Abbott, the built-in blood glucose meter found in the FreeStyle Libre 3 reader is not impacted by these ongoing concerns. It can still be used to check a patient’s glucose at any time.
Additional details about this recall from the FDA’s medical device recall database are available here.