Transcatheter Cardiovascular Therapeutics (TCT)
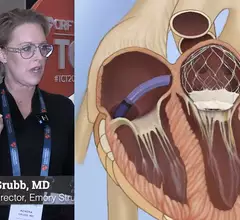
The Transcatheter Cardiovascular Therapeutics (TCT) conference is the Cardiovascular Research Foundation's (CRF) annual scientific symposium and the largest conference focused on interventional cardiovascular medicine. TCT includes seminars on all areas of intervention cardiology, structural heart, vascular in interventions, peripheral artery disease, and other procedures in the cath lab.
Displaying 33 - 40 of 123